Propylene glycol: Difference between revisions
→Miscellaneous applications: remove scare quotes |
moved links around |
||
| Line 128: | Line 128: | ||
* In the cosmetics industry, where PG is very commonly used as a carrier or base for various types of makeup.<ref>{{Cite journal|last=Fiume|first=Monice M.|last2=Bergfeld|first2=Wilma F.|last3=Belsito|first3=Donald V.|last4=Hill|first4=Ronald A.|last5=Klaassen|first5=Curtis D.|last6=Liebler|first6=Daniel|last7=Marks|first7=James G.|last8=Shank|first8=Ronald C.|last9=Slaga|first9=Thomas J.|date=September 2012|title=Safety assessment of propylene glycol, tripropylene glycol, and PPGs as used in cosmetics|journal=International Journal of Toxicology|volume=31|issue=5 Suppl|pages=245S–60S|doi=10.1177/1091581812461381|issn=1092-874X|pmid=23064775}}</ref> |
* In the cosmetics industry, where PG is very commonly used as a carrier or base for various types of makeup.<ref>{{Cite journal|last=Fiume|first=Monice M.|last2=Bergfeld|first2=Wilma F.|last3=Belsito|first3=Donald V.|last4=Hill|first4=Ronald A.|last5=Klaassen|first5=Curtis D.|last6=Liebler|first6=Daniel|last7=Marks|first7=James G.|last8=Shank|first8=Ronald C.|last9=Slaga|first9=Thomas J.|date=September 2012|title=Safety assessment of propylene glycol, tripropylene glycol, and PPGs as used in cosmetics|journal=International Journal of Toxicology|volume=31|issue=5 Suppl|pages=245S–60S|doi=10.1177/1091581812461381|issn=1092-874X|pmid=23064775}}</ref> |
||
* For [[pitfall trap|trapping]] and preserving insects (including as a [[DNA]] preservative).<ref>{{Cite journal|last=Rubin|first=Benjamin E. R.|last2=Czekanski-Moir|first2=Jesse E.|last3=Wray|first3=Brian D.|last4=Moreau|first4=Corrie S.|date=2013-03-13|title=DNA preservation: a test of commonly used preservatives for insects|journal=Invertebrate Systematics|language=en|volume=27|issue=1|pages=81–86|doi=10.1071/IS12067|issn=1447-2600}}</ref> |
* For [[pitfall trap|trapping]] and preserving insects (including as a [[DNA]] preservative).<ref>{{Cite journal|last=Rubin|first=Benjamin E. R.|last2=Czekanski-Moir|first2=Jesse E.|last3=Wray|first3=Brian D.|last4=Moreau|first4=Corrie S.|date=2013-03-13|title=DNA preservation: a test of commonly used preservatives for insects|journal=Invertebrate Systematics|language=en|volume=27|issue=1|pages=81–86|doi=10.1071/IS12067|issn=1447-2600}}</ref> |
||
* Along with [[Vegetable Glycerin|vegetable glycerin]] as the main ingredient (<1–92%) in [[e-liquid]] used in [[electronic cigarettes]], where it is [[aerosolized]] to resemble smoke. It serves as both the carrier for substances like nicotine and cannabinoids, as well as for creating a vapor which resembles smoke.<ref>{{cite web|url=http://www.health.harvard.edu/blog/electronic-cigarettes-help-or-hazard-201109223395|title=Electronic cigarettes: Help or hazard?|last1=Simon|first1=Harvey|date=2011-09-22|publisher=Harvard Medical School - Harvard Health Publications|accessdate=Sep 22, 2011}}</ref> |
* Along with [[Vegetable Glycerin|vegetable glycerin]] as the main ingredient (<1–92%) in [[e-liquid]] used in [[electronic cigarettes]], where it is [[aerosolized]] to resemble smoke. It serves as both the carrier for substances like [[nicotine]] and [[cannabinoids]], as well as for creating a vapor which resembles smoke.<ref>{{cite web|url=http://www.health.harvard.edu/blog/electronic-cigarettes-help-or-hazard-201109223395|title=Electronic cigarettes: Help or hazard?|last1=Simon|first1=Harvey|date=2011-09-22|publisher=Harvard Medical School - Harvard Health Publications|accessdate=Sep 22, 2011}}</ref> |
||
* For the creation of [[theatrical smoke and fog]] in special effects for film and live entertainment. So-called 'smoke machines' or 'hazers' vaporize a mixture of PG and water to create the illusion of smoke. While many of these machines use a PG-based fuel, some use oil. Those which use PG do so in a process which is identical to how electronic cigarettes work; utilizing a heating element to produce a dense vapor. The vapor produced by these machines has the aesthetic look and appeal of smoke, but without exposing performers and stage crew to the harms and odors associated with actual smoke.<ref>{{Cite web|url=https://nevadafilm.com/production-notes-haze-machines/|title=Production Notes: Haze Machines|last=Nevada Film Office|date=February 19, 2019|website=nevadafilm.com|url-status=live|archive-url=|archive-date=|access-date=November 1, 2019}}</ref><ref>{{Cite web|url=https://www.provideocoalition.com/atmosphere101|title=Atmosphere: Hazers, Fazers, Smoke and Fog 101 by Daniel Brea|last=Daniel|first=Brea|date=July 15, 2016|website=provideocoalition.com|url-status=live|archive-url=|archive-date=|access-date=November 1, 2019}}</ref> |
* For the creation of [[theatrical smoke and fog]] in special effects for film and live entertainment. So-called 'smoke machines' or 'hazers' vaporize a mixture of PG and water to create the illusion of smoke. While many of these machines use a PG-based fuel, some use oil. Those which use PG do so in a process which is identical to how electronic cigarettes work; utilizing a heating element to produce a dense vapor. The vapor produced by these machines has the aesthetic look and appeal of smoke, but without exposing performers and stage crew to the harms and odors associated with actual smoke.<ref>{{Cite web|url=https://nevadafilm.com/production-notes-haze-machines/|title=Production Notes: Haze Machines|last=Nevada Film Office|date=February 19, 2019|website=nevadafilm.com|url-status=live|archive-url=|archive-date=|access-date=November 1, 2019}}</ref><ref>{{Cite web|url=https://www.provideocoalition.com/atmosphere101|title=Atmosphere: Hazers, Fazers, Smoke and Fog 101 by Daniel Brea|last=Daniel|first=Brea|date=July 15, 2016|website=provideocoalition.com|url-status=live|archive-url=|archive-date=|access-date=November 1, 2019}}</ref> |
||
* As an additive in [[PCR]] to reduce the melting temperature of nucleic acids for targeting of [[GC rich]] sequences. |
* As an additive in [[PCR]] to reduce the melting temperature of nucleic acids for targeting of [[GC rich]] sequences. |
||
| Line 145: | Line 145: | ||
===Skin, eye and inhalation contact=== |
===Skin, eye and inhalation contact=== |
||
Propylene glycol is essentially non-irritating to the skin.<ref>{{Cite journal|author= Agency for Toxic Substances and Disease Registry |year= 2008|title=Addendum to the Toxicological Profile for Propylene Glycol |journal= |volume= |issue= |page= 7 |publisher= |doi= |pmid= |pmc= |url= }}</ref> Undiluted propylene glycol is minimally irritating to the eye, producing slight transient [[conjunctivitis]]; the eye recovers after the exposure is removed. A 2018 human volunteer study found that 10 male and female subjects undergoing 4 hours exposures to concentrations of up to 442 mg/m3 and 30 minutes exposures to concentrations of up to 871 mg/m3 in combination with moderate exercise did not show pulmonary function deficits, or signs of ocular irritation, with only slight symptoms of respiratory irritation reported.<ref>{{cite journal | title=Lack of respiratory and ocular effects following acute propylene glycol exposure in healthy humans. | author=Dalton P, Soreth B, Maute C, Novaleski C, and Banton M | journal=Inhal. Toxicol. | volume=30 | issue=3 | pages=124–132| year=2018| doi=10.1080/08958378.2018.1470207 | pmid=29764241 }}</ref> Inhalation of propylene glycol vapors appears to present no significant hazard in ordinary applications.<ref name="Robertson47">{{cite journal | url=http://jpet.aspetjournals.org/content/91/1/52.abstract | title=Tests for the chronic toxicity of propylene glycol and triethylene glycol on monkeys and rats by vapor inhalation and oral administration |author1=Robertson, OH |author2=Loosli, CG |author3=Puck, TT |author4=Wise, H |author5=Lemon, HM |author6=Lester, W | journal=JPET |date=September 1947 | volume=91 | issue=1 | pages=52–76 | pmid=20265820 | quote=air containing these vapors in amounts up to the saturation point is completely harmless}}</ref> Due to the lack of chronic inhalation data, it is recommended that propylene glycol not be used in inhalation applications such as theatrical productions, or antifreeze solutions for emergency [[eye wash]] stations.<ref>[http://msdssearch.dow.com/PublishedLiteratureDOWCOM/dh_091b/0901b8038091b508.pdf?filepath=propyleneglycol/pdfs/noreg/117-01682.pdf A Guide to Glycols], Dow, page 36</ref> Recently, propylene glycol (commonly alongside [[glycerol]]) has been included as a carrier for |
Propylene glycol is essentially non-irritating to the skin.<ref>{{Cite journal|author= Agency for Toxic Substances and Disease Registry |year= 2008|title=Addendum to the Toxicological Profile for Propylene Glycol |journal= |volume= |issue= |page= 7 |publisher= |doi= |pmid= |pmc= |url= }}</ref> Undiluted propylene glycol is minimally irritating to the eye, producing slight transient [[conjunctivitis]]; the eye recovers after the exposure is removed. A 2018 human volunteer study found that 10 male and female subjects undergoing 4 hours exposures to concentrations of up to 442 mg/m3 and 30 minutes exposures to concentrations of up to 871 mg/m3 in combination with moderate exercise did not show pulmonary function deficits, or signs of ocular irritation, with only slight symptoms of respiratory irritation reported.<ref>{{cite journal | title=Lack of respiratory and ocular effects following acute propylene glycol exposure in healthy humans. | author=Dalton P, Soreth B, Maute C, Novaleski C, and Banton M | journal=Inhal. Toxicol. | volume=30 | issue=3 | pages=124–132| year=2018| doi=10.1080/08958378.2018.1470207 | pmid=29764241 }}</ref> Inhalation of propylene glycol vapors appears to present no significant hazard in ordinary applications.<ref name="Robertson47">{{cite journal | url=http://jpet.aspetjournals.org/content/91/1/52.abstract | title=Tests for the chronic toxicity of propylene glycol and triethylene glycol on monkeys and rats by vapor inhalation and oral administration |author1=Robertson, OH |author2=Loosli, CG |author3=Puck, TT |author4=Wise, H |author5=Lemon, HM |author6=Lester, W | journal=JPET |date=September 1947 | volume=91 | issue=1 | pages=52–76 | pmid=20265820 | quote=air containing these vapors in amounts up to the saturation point is completely harmless}}</ref> Due to the lack of chronic inhalation data, it is recommended that propylene glycol not be used in inhalation applications such as theatrical productions, or antifreeze solutions for emergency [[eye wash]] stations.<ref>[http://msdssearch.dow.com/PublishedLiteratureDOWCOM/dh_091b/0901b8038091b508.pdf?filepath=propyleneglycol/pdfs/noreg/117-01682.pdf A Guide to Glycols], Dow, page 36</ref> Recently, propylene glycol (commonly alongside [[glycerol]]) has been included as a carrier for nicotine and other additives in [[e-cigarette liquids]], the use of which presents a novel form of exposure. The potential hazards of chronic inhalation of propylene glycol or the latter substance as a whole are as-yet unknown.{{citation needed|date=January 2019}} |
||
According to a 2010 study, the concentrations of PGEs (counted as the sum of propylene glycol and glycol ethers) in indoor air, particularly bedroom air, has been linked to increased risk of developing numerous respiratory and immune disorders in children, including [[asthma]], [[hay fever]], [[eczema]], and allergies, with increased risk ranging from 50% to 180%. This concentration has been linked to use of water-based paints and water-based system cleansers. However, the study authors write that [[glycol ethers]] and not propylene glycol are the likely culprit.<ref>{{cite news |url=https://www.sciencedaily.com/releases/2010/10/101019084607.htm |title=Everyday Substances Increase Risk of Allergies in Children, Swedish Study Reveals |work=ScienceDaily |date=Oct 19, 2010 }}</ref><ref>{{cite web|url=http://www.hsph.harvard.edu/news/features/features/paints-cleaners-children-asthma-allergy.html|title=Chemical Compounds Emitted From Common Household Paints and Cleaners Increase Risks of Asthma and Allergies in Children|publisher=Harvard|accessdate=3 October 2018}}</ref><ref>{{Cite journal |
According to a 2010 study, the concentrations of PGEs (counted as the sum of propylene glycol and glycol ethers) in indoor air, particularly bedroom air, has been linked to increased risk of developing numerous respiratory and immune disorders in children, including [[asthma]], [[hay fever]], [[eczema]], and allergies, with increased risk ranging from 50% to 180%. This concentration has been linked to use of water-based paints and water-based system cleansers. However, the study authors write that [[glycol ethers]] and not propylene glycol are the likely culprit.<ref>{{cite news |url=https://www.sciencedaily.com/releases/2010/10/101019084607.htm |title=Everyday Substances Increase Risk of Allergies in Children, Swedish Study Reveals |work=ScienceDaily |date=Oct 19, 2010 }}</ref><ref>{{cite web|url=http://www.hsph.harvard.edu/news/features/features/paints-cleaners-children-asthma-allergy.html|title=Chemical Compounds Emitted From Common Household Paints and Cleaners Increase Risks of Asthma and Allergies in Children|publisher=Harvard|accessdate=3 October 2018}}</ref><ref>{{Cite journal |
||
Revision as of 16:19, 14 March 2020

| |||
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
Propane-1,2-diol | |||
| Other names
Propylene glycol
α-Propylene glycol 1,2-Propanediol 1,2-Dihydroxypropane Methyl ethyl glycol Methylethylene glycol | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.000.307 | ||
| EC Number |
| ||
| E number | E1520 (additional chemicals) | ||
PubChem CID
|
|||
| RTECS number |
| ||
| UNII | |||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| C3H8O2 | |||
| Molar mass | 76.095 g·mol−1 | ||
| Density | 1.036 g/cm3 | ||
| Melting point | −59 °C (−74 °F; 214 K) | ||
| Boiling point | 188.2 °C (370.8 °F; 461.3 K) | ||
| Miscible | |||
| Solubility in ethanol | Miscible | ||
| Solubility in diethyl ether | Miscible | ||
| Solubility in acetone | Miscible | ||
| Solubility in chloroform | Miscible | ||
| log P | -1.34[2] | ||
| Thermal conductivity | 0.34 W/m-K (50% H2O @ 90 °C (194 °F)) | ||
| Viscosity | 0.042 Pa·s | ||
| Pharmacology | |||
| QA16QA01 (WHO) | |||
| Hazards | |||
| NFPA 704 (fire diamond) | |||
| Related compounds | |||
Related glycols
|
Ethylene glycol, 1,3-propanediol | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Propylene glycol (IUPAC name: propane-1,2-diol) is an organic compound with the chemical formula CH3CH(OH)CH2OH. It is a viscous, colorless liquid, which is nearly odorless but possesses a faintly sweet taste. Containing two alcohol groups, it is classed as a diol. It is miscible with a broad range of solvents, including water, acetone, and chloroform. In general, glycols are non-irritating, have very low volatility and very low toxicity.[4]
It is produced on a large scale primarily for the production of polymers. In the European Union, it has the E-number E1520 for food applications. For cosmetics and pharmacology, the number is E490. Propylene glycol is also present in propylene glycol alginate, which is known as E405. Propylene glycol is a compound which is generally recognized as safe (GRAS) by the U.S. Food and Drug Administration (FDA) under 21 CFR x184.1666 and is also approved by FDA for certain uses as an indirect food additive. Propylene glycol is approved and used as a vehicle for topical, oral and some intravenous pharmaceutical preparations in U.S. and in Europe.
Structure
The compound is sometimes called (alpha) α-propylene glycol to distinguish it from the isomer propane-1,3-diol, known as (beta) β-propylene glycol. Propylene glycol is chiral. Commercial processes typically use the racemate. The S-isomer is produced by biotechnological routes.
Production
Industrial
Industrially, propylene glycol is mainly produced from propylene oxide (for food-grade use). According to a 2018 source, 2.16 M tonnes are produced annually.[4] Manufacturers use either non-catalytic high-temperature process at 200 °C (392 °F) to 220 °C (428 °F), or a catalytic method, which proceeds at 150 °C (302 °F) to 180 °C (356 °F) in the presence of ion exchange resin or a small amount of sulfuric acid or alkali.[5]

Final products contain 20% propylene glycol, 1.5% of dipropylene glycol, and small amounts of other polypropylene glycols.[6] Further purification produces finished industrial grade or USP/JP/EP/BP grade propylene glycol that is typically 99.5% or greater.
Propylene glycol can also be obtained from glycerol, a byproduct from the production of biodiesel.[4] This starting material is usually reserved for industrial use because of the noticeable odor and taste that accompanies the final product.
Laboratory
S-Propanediol is synthesized from via fermentation methods. Lactic acid and lactaldehyde are common intermediates. Dihydroxyacetone phosphate, one of the two products of breakdown (glycolysis) of fructose 1,6-bisphosphate, is a precursor to methylglyoxal. This conversion is the basis of a potential biotechnological route to the commodity chemical 1,2-propanediol. Three-carbon deoxysugars are also precursor to the 1,2-diol.[4]
A small-scale, nonbiological route from D-mannitol is illustrated in the following scheme:[7]
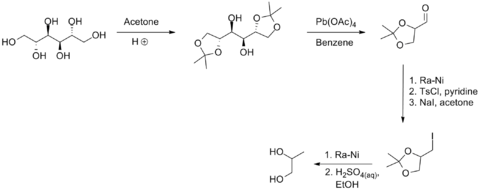
Applications
Polymers
Forty-five percent of propylene glycol produced is used as chemical feedstock for the production of unsaturated polyester resins. In this regard, propylene glycol reacts with a mixture of unsaturated maleic anhydride and isophthalic acid to give a copolymer. This partially unsaturated polymer undergoes further crosslinking to yield thermoset plastics. Related to this application, propylene glycol reacts with propylene oxide to give oligomers and polymers that are used to produce polyurethanes.[4] Propylene glycol is used in waterbased acrylic architectural paints to extend dry time which it accomplishes by preventing the surface from drying due to its slower evaporation rate compared to water.
Food
Propylene glycol is also used in various edible items such as coffee-based drinks, liquid sweeteners, ice cream, whipped dairy products and soda.[8][9] Vaporizers used for delivery of pharmaceuticals or personal-care products often include propylene glycol among the ingredients.[4] In alcohol-based hand sanitizers, it is used as a humectant to prevent the skin from drying.[10] Propylene glycol is used as a solvent in many pharmaceuticals, including oral, injectable, and topical formulations. Many pharmaceutical drugs which are insoluble in water utilize PG as a solvent and carrier; benzodiazepine tablets are one example.[11] PG is also used as a solvent and carrier for many pharmaceutical capsule preparations. Additionally, certain formulations of artificial tears use proplyene glycol as an ingredient.[12]

Antifreeze
The freezing point of water is depressed when mixed with propylene glycol. It is used as aircraft de-icing fluid.[4][13] Water-propylene glycol mixtures dyed pink to indicate the mixture is relatively nontoxic are sold under the name of RV or marine antifreeze. Propylene glycol is frequently used as a substitute for ethylene glycol in low toxicity, environmentally friendly automotive antifreeze. It is also used to winterize the plumbing systems in vacant structures.[14] The eutectic composition/temperature is 60:40 propylene glycol:water/-60 °C.[15][16] The −50 °F/−45 °C commercial product is, however, water rich; a typical formulation is 40:60.[17]
Miscellaneous applications

Propylene glycol (often abbreviated 'PG') has many applications. Some common applications see PG used:
- As a solvent for many substances, both natural and synthetic.[18]
- As a humectant (E1520).
- In veterinary medicine as an oral treatment for hyperketonaemia in ruminants.[19]
- In the cosmetics industry, where PG is very commonly used as a carrier or base for various types of makeup.[20]
- For trapping and preserving insects (including as a DNA preservative).[21]
- Along with vegetable glycerin as the main ingredient (<1–92%) in e-liquid used in electronic cigarettes, where it is aerosolized to resemble smoke. It serves as both the carrier for substances like nicotine and cannabinoids, as well as for creating a vapor which resembles smoke.[22]
- For the creation of theatrical smoke and fog in special effects for film and live entertainment. So-called 'smoke machines' or 'hazers' vaporize a mixture of PG and water to create the illusion of smoke. While many of these machines use a PG-based fuel, some use oil. Those which use PG do so in a process which is identical to how electronic cigarettes work; utilizing a heating element to produce a dense vapor. The vapor produced by these machines has the aesthetic look and appeal of smoke, but without exposing performers and stage crew to the harms and odors associated with actual smoke.[23][24]
- As an additive in PCR to reduce the melting temperature of nucleic acids for targeting of GC rich sequences.
Safety in humans
When used in average quantities, propylene glycol has no measurable effect on development and/or reproduction on animals and probably does not adversely affect human development or reproduction.[25] The safety of electronic cigarettes—which utilize PG-based preparations of nicotine or THC and other cannabinoids—is the subject of much controversy.[26][27][28]
Oral administration
The acute oral toxicity of propylene glycol is very low, and large quantities are required to cause perceptible health effects in humans; in fact, propylene glycol is three times less toxic than ethanol.[29] Propylene glycol is metabolized in the human body into pyruvic acid (a normal part of the glucose-metabolism process, readily converted to energy), acetic acid (handled by ethanol-metabolism), lactic acid (a normal acid generally abundant during digestion),[30] and propionaldehyde (a potentially hazardous substance).[31][32][33] According to the Dow Chemical Company, The LD50 (Lethal Dose that kills in 50% of tests) for rats is 20 g/kg (rat/oral).[34][35]
Toxicity generally occurs at plasma concentrations over 4 g/L, which requires extremely high intake over a relatively short period of time, or when used as a vehicle for drugs or vitamins given intravenously or orally in large bolus doses.[36] It would be nearly impossible to reach toxic levels by consuming foods or supplements, which contain at most 1 g/kg of PG, except for alcoholic beverages in the US which are allowed 5 percent = 50g/kg.[37] Cases of propylene glycol poisoning are usually related to either inappropriate intravenous administration or accidental ingestion of large quantities by children.[38]
The potential for long-term oral toxicity is also low. In an NTP continuous breeding study, no effects on fertility were observed in male or female mice that received propylene glycol in drinking water at doses up to 10,100 mg/kg bw/day. No effects on fertility were seen in either the first or second generation of treated mice.[25] In a 2-year study, 12 rats were provided with feed containing as much as 5% propylene glycol, and showed no apparent ill effects.[39] Because of its low chronic oral toxicity, propylene glycol was classified by the U. S. Food and Drug Administration as "generally recognized as safe" (GRAS) for use as a direct food additive, including frozen foods such as ice cream and frozen desserts.[37][40] The GRAS designation is specific to its use in food, and does not apply to other uses.[41]
Skin, eye and inhalation contact
Propylene glycol is essentially non-irritating to the skin.[42] Undiluted propylene glycol is minimally irritating to the eye, producing slight transient conjunctivitis; the eye recovers after the exposure is removed. A 2018 human volunteer study found that 10 male and female subjects undergoing 4 hours exposures to concentrations of up to 442 mg/m3 and 30 minutes exposures to concentrations of up to 871 mg/m3 in combination with moderate exercise did not show pulmonary function deficits, or signs of ocular irritation, with only slight symptoms of respiratory irritation reported.[43] Inhalation of propylene glycol vapors appears to present no significant hazard in ordinary applications.[44] Due to the lack of chronic inhalation data, it is recommended that propylene glycol not be used in inhalation applications such as theatrical productions, or antifreeze solutions for emergency eye wash stations.[45] Recently, propylene glycol (commonly alongside glycerol) has been included as a carrier for nicotine and other additives in e-cigarette liquids, the use of which presents a novel form of exposure. The potential hazards of chronic inhalation of propylene glycol or the latter substance as a whole are as-yet unknown.[citation needed]
According to a 2010 study, the concentrations of PGEs (counted as the sum of propylene glycol and glycol ethers) in indoor air, particularly bedroom air, has been linked to increased risk of developing numerous respiratory and immune disorders in children, including asthma, hay fever, eczema, and allergies, with increased risk ranging from 50% to 180%. This concentration has been linked to use of water-based paints and water-based system cleansers. However, the study authors write that glycol ethers and not propylene glycol are the likely culprit.[46][47][48]
Propylene glycol has not caused sensitization or carcinogenicity in laboratory animal studies, nor has it demonstrated genotoxic potential.[49][50]
Intravenous administration
Studies with intravenously administered propylene glycol have resulted in LD50 values in rats and rabbits of 7 mL/kg BW.[51] Ruddick (1972) also summarized intramuscular LD50 data for rat as 13-20 mL/kg BW, and 6 mL/kg BW for the rabbit. Adverse effects to intravenous administration of drugs that use propylene glycol as an excipient have been seen in a number of people, particularly with large bolus dosages. Responses may include CNS depression, "hypotension, bradycardia, QRS and T abnormalities on the ECG, arrhythmia, cardiac arrhythmias, seizures, agitation, serum hyperosmolality, lactic acidosis, and haemolysis".[52] A high percentage (12% to 42%) of directly-injected propylene glycol is eliminated or secreted in urine unaltered depending on dosage, with the remainder appearing in its glucuronide-form. The speed of renal filtration decreases as dosage increases,[53] which may be due to propylene glycol's mild anesthetic / CNS-depressant -properties as an alcohol.[54] In one case, intravenous administration of propylene glycol-suspended nitroglycerin to an elderly man may have induced coma and acidosis.[55] However, no confirmed lethality from propylene glycol was reported.
Animals
Propylene glycol is an approved food additive for dog and sugar glider food under the category of animal feed and is generally recognized as safe for dogs,[56] with an LD50 of 9 mL/kg. The LD50 is higher for most laboratory animals (20 mL/kg).[57] However, it is prohibited for use in food for cats due to links to Heinz body formation and a reduced lifespan of red blood cells.[58] Heinz body formation from MPG has not been observed in dogs, cattle, or humans.
Allergic reaction
Estimates on the prevalence of propylene glycol allergy range from 0.8% (10% propylene glycol in aqueous solution) to 3.5% (30% propylene glycol in aqueous solution).[59][60][61] The North American Contact Dermatitis Group (NACDG) data from 1996 to 2006 showed that the most common site for propylene glycol contact dermatitis was the face (25.9%), followed by a generalized or scattered pattern (23.7%).[59] Investigators believe that the incidence of allergic contact dermatitis to propylene glycol may be greater than 2% in patients with eczema or fungal infections, which are very common in countries with lesser sun exposure and lower-than-normal vitamin D balances. Therefore, propylene glycol allergy is more common in those countries.[62]
Because of its potential for allergic reactions and frequent use across a variety of topical and systemic products, propylene glycol was named the American Contact Dermatitis Society's Allergen of the Year for 2018.[63][64] Recent publication from The Mayo Clinic reported 0.85% incidence of positive patch tests to propylene glycol (100/11,738 patients) with an overall irritant rate of 0.35% (41/11,738 patients) during a 20-year period of 1997–2016.[65] 87% of the reactions were classified as weak and 9% as strong. The positive reaction rates were 0%, 0.26%, and 1.86% for 5%, 10%, and 20% propylene glycol respectively, increasing with each concentration increase. The irritant reaction rates were 0.95%, 0.24%, and 0.5% for 5%, 10%, and 20% propylene glycol, respectively. Propylene glycol skin sensitization occurred in patients sensitive to a number of other concomitant positive allergens, most common of which were: Myroxylon pereirae resin, benzalkonium chloride, carba mix, potassium dichromate, neomycin sulfate; for positive propylene glycol reactions, the overall median of 5 and mean of 5.6 concomitant positive allergens was reported.
Environmental
Propylene glycol is expected to degrade rapidly in water from biological processes, but is not expected to be significantly influenced by hydrolysis, oxidation, volatilization, bioconcentration, or adsorption to sediment.[66] Propylene glycol is readily biodegradable under aerobic conditions in freshwater, in seawater and in soil. Therefore, propylene glycol is considered as not persistent in the environment.
Propylene glycol exhibits a low degree of toxicity toward aquatic organisms. There are several guideline studies available for freshwater fish with the lowest observed effect concentration of 96-h LC50 value of 40,613 mg/l in a study with Oncorhynchus mykiss. Similarly, the effect concentration determined in marine fish is a 96-h LC50 of >10,000 mg/l in Scophthalmus maximus.
References
- ^ The Merck Index: An Encyclopedia of Chemicals, Drugs, and Biologicals. Merck & Co. 1989. ISBN 978-0911910285.
- ^ "Propylene Glycol_msds".
- ^ "PROPYLENE GLYCOL - CAMEO Chemicals". NOAA Office of Response and Restoration. NOAA. Retrieved 3 October 2018.
- ^ a b c d e f g Sullivan, Carl J.; Kuenz, Anja; Vorlop, Klaus‐Dieter (2018). "Propanediols". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a22_163.pub2. ISBN 978-3527306732.
- ^ Chauvel, Alain; Lefebvre, Gilles (1989). Petrochemical Processes. Vol. Volume 2: Major Oxygenated, Chlorinated and Nitrated Derivatives. Editions Technip. p. 26. ISBN 9782710805632.
{{cite book}}:|volume=has extra text (help) - ^ "1,2-propanediol: chemical product info at CHEMINDUSTRY.RU". Retrieved 3 October 2018.
- ^ Hanessian, Stephen (1983). Total Synthesis of Natural Products: The 'Chiron' Approach. Pergamon press. p. 41. ISBN 978-0080307152.
- ^ "Quackmail: Why You Shouldn't Fall For The Internet's Newest Fool, The Food Babe". Butterworth, Trevor. Forbes. 16 June 2014. Retrieved 18 March 2015.
- ^ G. Jackson, R. T. Roberts and T. Wainwright (January 1980). "Mechanism of Beer Foam Stabilization by Propylene Glycol Alginate". Journal of the Institute of Brewing. 86 (1): 34–37. doi:10.1002/j.2050-0416.1980.tb03953.x.
- ^ Lohrey, Jackie. "Ingredients in Hand Sanitizer". LIVESTRONG.COM. Retrieved 2018-06-11.
- ^ Janusz Szajewski, MD, Warsaw Poison Control Centre (August 1991). "Propylene glycol (PIM 443)". IPCS INChem. Retrieved July 2, 2009.
- ^ Pucker AD, Ng SM, Nichols JJ (2016). "Over the counter (OTC) artificial tear drops for dry eye syndrome". Cochrane Database Syst Rev. 2: CD009729. doi:10.1002/14651858.CD009729.pub2. PMC 5045033. PMID 26905373.
- ^ "What's That Stuff? Aircraft Deicers". Chemical & Engineering News. American Chemical Society. 2000-07-10. Retrieved 2013-06-21.
- ^ "5 Ways to Winterize a Vacant Home". wikiHow. 2012-06-11. Retrieved 2014-02-27.
- ^ "Properties of Some Particular Solutions" (PDF). Portal del DMT. Retrieved 2014-02-27.
- ^ Salnick, Robert (2010-08-04). "Windborne in Puget Sound: Why does a holding plate work?". Windborneinpugetsound.blogspot.com. Retrieved 2014-02-27.
- ^ "Material Safety Data Sheet: Winter Care RV Antifreeze" (PDF). Chemical Specialties. Retrieved 2014-02-27.
- ^ Bradley, Jean-Claude; Abraham, Michael H; Acree, William E; Lang, Andrew (2015). "Predicting Abraham model solvent coefficients". Chemistry Central Journal. 9 (1): 12. doi:10.1186/s13065-015-0085-4. ISSN 1752-153X. PMC 4369285. PMID 25798192.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Nielsen, Nicolaj (2004). "Propylene glycol for dairy cows". Animal Feed Science and Technology. 115 (3–4): 191–213. doi:10.1016/j.anifeedsci.2004.03.008.
- ^ Fiume, Monice M.; Bergfeld, Wilma F.; Belsito, Donald V.; Hill, Ronald A.; Klaassen, Curtis D.; Liebler, Daniel; Marks, James G.; Shank, Ronald C.; Slaga, Thomas J. (September 2012). "Safety assessment of propylene glycol, tripropylene glycol, and PPGs as used in cosmetics". International Journal of Toxicology. 31 (5 Suppl): 245S–60S. doi:10.1177/1091581812461381. ISSN 1092-874X. PMID 23064775.
- ^ Rubin, Benjamin E. R.; Czekanski-Moir, Jesse E.; Wray, Brian D.; Moreau, Corrie S. (2013-03-13). "DNA preservation: a test of commonly used preservatives for insects". Invertebrate Systematics. 27 (1): 81–86. doi:10.1071/IS12067. ISSN 1447-2600.
- ^ Simon, Harvey (2011-09-22). "Electronic cigarettes: Help or hazard?". Harvard Medical School - Harvard Health Publications. Retrieved Sep 22, 2011.
- ^ Nevada Film Office (February 19, 2019). "Production Notes: Haze Machines". nevadafilm.com. Retrieved November 1, 2019.
{{cite web}}: CS1 maint: url-status (link) - ^ Daniel, Brea (July 15, 2016). "Atmosphere: Hazers, Fazers, Smoke and Fog 101 by Daniel Brea". provideocoalition.com. Retrieved November 1, 2019.
{{cite web}}: CS1 maint: url-status (link) - ^ a b National Toxicology Program NIEHS (2004). "NTP-CERHR Monograph on the Potential Human Reproductive and Developmental Effects of Propylene Glycol". NIH Publication No. 04-4482.
{{cite journal}}: Cite journal requires|journal=(help) - ^ CDC (March 11, 2019). "Electronic Cigarettes".
{{cite web}}: CS1 maint: url-status (link) - ^ Havelka, Jacqueline (April 27, 2017). "Is Vaping Safe?". leafly.com.
{{cite web}}: CS1 maint: url-status (link) - ^ Peki, Winston (May 5, 2019). "Are Vaporizers Safe?". herbonaut.com.
{{cite web}}: CS1 maint: url-status (link) - ^ Lehman A, Newman H (1937). "Propylene glycol: Rate of metabolism absorption, and excretion, with a method for estimation in body fluids". J Pharmacol Exp Ther. 60: 312–322.
- ^ Hamilton, D. J. (1890). "Gastric Dyspepsia". The Lancet. 2 (3493): 306. doi:10.1016/S0140-6736(02)17110-8.
- ^ "Material Safety Data Sheet Propionaldehyde MSDS". ScienceLab.com. 2010.
- ^ Miller DN, Bazzano G; Bazzano (1965). "Propanediol metabolism and its relation to lactic acid metabolism". Ann NY Acad Sci. 119 (3): 957–973. Bibcode:1965NYASA.119..957M. doi:10.1111/j.1749-6632.1965.tb47455.x. PMID 4285478.
- ^ Ruddick JA (1972). "Toxicology, metabolism, and biochemistry of 1,2-propanediol". Toxicol Appl Pharmacol. 21 (1): 102–111. doi:10.1016/0041-008X(72)90032-4. PMID 4553872.
- ^ rocklinusd. "Lethal dose table" (PDF).
{{cite journal}}: Cite journal requires|journal=(help) - ^ Alton E. Martin - Frank H. Murphy DOW CHEMICAL COMPANY. "GLYCOLS - PROPYLENE GLYCOLS" (PDF).
{{cite journal}}: Cite journal requires|journal=(help) - ^ Flanagan RJ; Braithwaite RA; Brown SS; Widdop B; de Wolff FA. The International Programme on Chemical Safety: Basic Analytical Toxicology. WHO, 1995.
- ^ a b U.S. Food and Drug Administration (FDA). "Subchapter B - Food for Human Consumption. § 184.1666. Propylene glycol." Code of Federal Regulations, 21 CFR 184.1666
- ^ National Library of Medicine;.Propylene glycol is used in antifreezes Human Toxicity Excerpts: CAS Registry Number: 57-55-6 (1,2-Propylene Glycol). Selected toxicity information from HSDB. 2005.
- ^ Gaunt, I. F.; Carpanini, F. M. B.; Grasso, P.; Lansdown, A. B. G. (1972). "Long-term toxicity of propylene glycol in rats". Food and Cosmetics Toxicology. 10 (2): 151–162. doi:10.1016/S0015-6264(72)80193-7.
- ^ "Database of Select Committee on GRAS Substances (SCOGS) Reviews". FDA. Retrieved 2016-05-11.
- ^ FDA. "Subchapter B - Food for Human Consumption. § 182.1. Substances that are generally recognized as safe." Code of Federal Regulations, 21 CFR 182.1
- ^ Agency for Toxic Substances and Disease Registry (2008). "Addendum to the Toxicological Profile for Propylene Glycol": 7.
{{cite journal}}: Cite journal requires|journal=(help) - ^ Dalton P, Soreth B, Maute C, Novaleski C, and Banton M (2018). "Lack of respiratory and ocular effects following acute propylene glycol exposure in healthy humans". Inhal. Toxicol. 30 (3): 124–132. doi:10.1080/08958378.2018.1470207. PMID 29764241.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Robertson, OH; Loosli, CG; Puck, TT; Wise, H; Lemon, HM; Lester, W (September 1947). "Tests for the chronic toxicity of propylene glycol and triethylene glycol on monkeys and rats by vapor inhalation and oral administration". JPET. 91 (1): 52–76. PMID 20265820.
air containing these vapors in amounts up to the saturation point is completely harmless
- ^ A Guide to Glycols, Dow, page 36
- ^ "Everyday Substances Increase Risk of Allergies in Children, Swedish Study Reveals". ScienceDaily. Oct 19, 2010.
- ^ "Chemical Compounds Emitted From Common Household Paints and Cleaners Increase Risks of Asthma and Allergies in Children". Harvard. Retrieved 3 October 2018.
- ^ Choi, Hyunok; Norbert Schmidbauer; Jan Sundell; Mikael Hasselgren; John Spengler; Carl-Gustaf Bornehag (2010-10-18). Hartl, Dominik (ed.). "Common Household Chemicals and the Allergy Risks in Pre-School Age Children". PLoS ONE. 5 (10): e13423. Bibcode:2010PLoSO...513423C. doi:10.1371/journal.pone.0013423. PMC 2956675. PMID 20976153.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ 1,2-Dihydroxypropane SIDS Initial Assessment Profile <"Archived copy" (PDF). Archived from the original (PDF) on 2009-02-19. Retrieved 2008-01-08.
{{cite web}}: CS1 maint: archived copy as title (link)>, UNEP Publications, SIAM 11, U.S.A, January 23–26, 2001, page 21. - ^ Title 21, U.S. Code of Federal Regulations. 1999.
- ^ Ruddick (1972). "Toxicology, metabolism, and biochemistry of 1,2-propanediol". Toxicol Appl Pharmacol. 21 (1): 102–111. doi:10.1016/0041-008X(72)90032-4. PMID 4553872.
- ^ Szajewski, Janusz. "Propylene Glycol (PIM 443)." 1991. 2 June 2010 http://www.inchem.org/documents/pims/chemical/pim443.htm#SectionTitle:9.1%20%20Acute%20poisoning
- ^ Speth, P. A. J.; Vree, T. B.; Neilen, N. F. M.; De Mulder, P. H. M.; Newell, D. R.; Gore, M. E.; De Pauw, B. E. (1987). "Propylene Glycol Pharmacokinetics and Effects after Intravenous Infusion in Humans". Therapeutic Drug Monitoring. 9 (3): 255–258. doi:10.1097/00007691-198709000-00001. PMID 3672566.
- ^ Seidenfeld, M. A.; Hanzlik, P. J. (1932). "The general properties, actions, and toxicity of propylene glycol". J Pharmacol Exp Ther. 44: 109–121.
- ^ Demey, H.; Daelemans, R.; De Broe, M.E.; Bossaert, L. (1984). "Propylene glycol intoxication due to intravenous nitroglycerin". The Lancet. 323 (8390): 1360. doi:10.1016/S0140-6736(84)91860-9. ISSN 0140-6736. PMID 6145062.
- ^ FDA. "Subchapter E - Animal Drugs, Feeds, and Related Products; § 582.1666. Propylene glycol." Code of Federal Regulations, 21 CFR 582.1666
- ^ Peterson, Michael; Talcott, Patricia A. (2006). Small animal toxicology. St. Louis: Saunders Elsevier. p. 997. ISBN 978-0-7216-0639-2.
- ^ "Propylene glycol and cats" (PDF). Retrieved 2013-06-21.
- ^ a b Warshaw, Erin M.; Botto, Nina C.; Maibach, Howard I.; Fowler, Joseph F.; Rietschel, Robert L.; Zug, Kathryn A.; Belsito, Donald V.; Taylor, James S.; DeLeo, Vincent A. (January 2009). "Positive patch-test reactions to propylene glycol: a retrospective cross-sectional analysis from the North American Contact Dermatitis Group, 1996 to 2006". Dermatitis: Contact, Atopic, Occupational, Drug. 20 (1): 14–20. doi:10.2310/6620.2008.08039. ISSN 2162-5220. PMID 19321115.
- ^ Lessmann, Holger; Schnuch, Axel; Geier, Johannes; Uter, Wolfgang (November 2005). "Skin-sensitizing and irritant properties of propylene glycol". Contact Dermatitis. 53 (5): 247–259. doi:10.1111/j.0105-1873.2005.00693.x. ISSN 0105-1873. PMID 16283903.
- ^ Wetter, David A.; Yiannias, James A.; Prakash, Amy V.; Davis, Mark D. P.; Farmer, Sara A.; el-Azhary, Rokea A. (November 2010). "Results of patch testing to personal care product allergens in a standard series and a supplemental cosmetic series: an analysis of 945 patients from the Mayo Clinic Contact Dermatitis Group, 2000-2007". Journal of the American Academy of Dermatology. 63 (5): 789–798. doi:10.1016/j.jaad.2009.11.033. ISSN 1097-6787. PMID 20643495.
- ^ American Medical Association, Council on Drugs (1994). AMA Drug Evaluations Annual 1994: 1224.
{{cite journal}}: Missing or empty|title=(help) - ^ Jacob, Sharon E.; Scheman, Andrew; McGowan, Maria A. (January–February 2018). "Propylene Glycol". Dermatitis: Contact, Atopic, Occupational, Drug. 29 (1): 3–5. doi:10.1097/DER.0000000000000315. ISSN 2162-5220. PMID 29059092.
- ^ "Allergen of the year may be nearer than you think". www.mdedge.com. Retrieved 2019-04-08.
- ^ Lalla SC, Nguyen H, Chaudhry H, Killian JM, Drage LA, Davis MDP, Yiannias JA, Hall MR. (2018). "Patch Testing to Propylene Glycol: The Mayo Clinic Experience". Dermatitis. 29 (4): 200–205. doi:10.1097/DER.0000000000000393. PMID 29923851.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ 1,2-Dihydroxypropane SIDS Initial Assessment Profile <"Archived copy" (PDF). Archived from the original (PDF) on 2009-02-19. Retrieved 2008-01-08.
{{cite web}}: CS1 maint: archived copy as title (link)>, UNEP Publications, SIAM 11, U.S.A, January 23–26, 2001, page 21.
External links
- Propylene glycol website
- WebBook page for C3H8O2
- ATSDR - Case Studies in Environmental Medicine: Ethylene Glycol and Propylene Glycol Toxicity U.S. Department of Health and Human Services (public domain)
- Propylene Glycol - chemical product info: properties, production, applications.
- ChemSub Online: Propylene glycol