Capsaicin
| Capsaicin | |
|---|---|
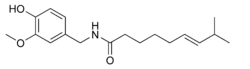
| |
| Systematic name | (E)-N- (4-hydroxy- 3-methoxybenzyl) - 8-methylnon-6-enamide |
| Molecular formula | C18H27NO3 |
| SMILES | CC(C)/C=C/CCCCC(NCC1=CC (OC)=C(O)C=C1)=O |
| CAS number | [404-86-4] |
| Molecular mass | 305.41 g/mol |
| Melting point | 62 - 65 °C |
The chemical compound capsaicin (8-methyl-N-vanillyl-6-nonenamide) is the active component of chili peppers, which are plants belonging to the genus Capsicum. It is an irritant for mammals, including humans, and produces a sensation of burning in any tissue it comes in contact with. Capsaicin and several related compounds are called capsaicinoids and are produced as a secondary metabolite by chili peppers, probably as deterrents against herbivores. Pure capsaicin is a hydrophobic, colorless, odorless, crystalline to waxy compound.
Capsaicinoids
Capsaicin is the main capsaicinoid in chili peppers, followed by dihydrocapsaicin. These two compounds are also about twice as potent to the taste and nerves as the minor capsaicinoids nordihydrocapsaicin, homodihydrocapsaicin, and homocapsaicin. Dilute solutions of pure capsaicinoids produced different types of pungency; however, these differences were not noted using more concentrated solutions.
| Capsaicinoid name | Abbrev. | Typical relative amount |
Scoville heat units |
Chemical structure |
|---|---|---|---|---|
| Capsaicin | C | 69% | 15,000,000 | 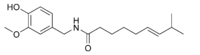
|
| Dihydrocapsaicin | DHC | 22% | 15,000,000 | 
|
| Nordihydrocapsaicin | NDHC | 7% | 9,100,000 | 
|
| Homodihydrocapsaicin | HDHC | 1% | 8,600,000 | 
|
| Homocapsaicin | HC | 1% | 8,600,000 | 
|
Natural function
Capsaicin is present in large quantities in the seeds and fleshy fruits of plants in the genus Capsicum. Such fruits have typically evolved to aid in seed dispersal by attracting animals, which consume the fruits and swallow the seeds, which pass through the digestive tract and are subsequently deposited elsewhere. Thus, it may seem paradoxical that a plant would expend the considerable amount of resources necessary to produce large, juicy fruits, only to fill them with a compound that acts as a powerful deterrent.
The seeds of Capsicum plants, however, are predominantly dispersed by birds, which, unlike mammals, are not sensitive to capsaicin. Chili pepper seeds consumed by birds pass through the digestive tract unharmed, whereas those consumed by mammals do not germinate at all. The presence of capsaicin in the fruits therefore protects them from being consumed by mammals, which wouldn't provide any benefit to the plant, while allowing them to be eaten by its preferred seed carriers.
Uses
Food
Because of the burning sensation caused by capsaicin when it comes in contact with human flesh, it is commonly used in food products to give them added spice or "heat" (piquancy). The degree of heat found within a food is measured on the Scoville scale. Typically the capsaicin is obtained from chili peppers. Hot sauce is an example of a product customarily containing large amounts of capsaicin and may contain chili peppers or pure capsaicin.
Capsaicin is a nonpolar molecule, and is therefore hydrophobic. Consequently, drinking water to reduce the burning caused by the molecule is ineffective, as the nonpolar capsaicin is unable to dissolve in the polar water molecules, and is instead spread across the surface of the mouth. This works by the same principle that causes oil and water to separate.
Instead, consuming foods high in fats and oils, such as milk or bread and butter, will help alleviate the burning. The lipophilic capsaicin is able to mix freely with the fats in the food and is removed from the surface of the mouth. Alcohol and alcoholic beverages also dissolve capsaicin due to the solvent characteristics of ethanol. Of course, over time the capsaicin will dissipate on its own.
Medical
Capsaicin is currently used in topical ointments to relieve the pain of peripheral neuropathy such as post-herpetic neuralgia caused by shingles. It may be used in concentrations of between 0.025% and 0.075%.
It may also be used as a cream for the temporary relief of minor aches and pains of muscles and joints associated with arthritis, simple backache, strains and sprains. The treatment typically involves the application of a topical anesthetic until the area is numb. Then the capsaicin is applied by a therapist wearing rubber gloves and a face mask. The capsaicin remains on the skin until the patient starts to feel the "heat", at which point it is promptly removed. Capsaicin is also available in large adhesive bandages that can be applied to the back.
The result appears to be that the nerves are overwhelmed from the burning sensation and are unable to report pain for an extended period of time. With chronic exposure to capsaicin, neurons are depleted of neurotransmitters and it leads to reduction in sensation of pain and blockade of neurogenic inflammation. If capsaicin is removed, the neurons recover.
Capsaicin is being explored as a cure for diabetes by researchers in Toronto, Canada. Early work curing diabetic mice looks promising. Capsaicin was injected into pancreatic sensory nerves of mice with Type 1 diabetes because of a suspected link between the nerves and diabetes.
Non-lethal force
Capsaicin is also the active ingredient in the chemical riot control agent pepper spray. When the spray comes in contact with skin, especially eyes or mucous membranes, it is very painful. Refer to the Scoville scale for a comparison of pepper spray to other sources of capsaicin.
In large quantities, capsaicin can cause death. Symptoms of overdose include difficulty breathing, blue skin, and convulsions. The large amount needed to kill an adult human and the low concentration of capsaicin in chilis make accidental poisoning by chili consumption exceedingly unlikely.
Possible drug abuse deterrent
Clifford Woolf, the Richard J. Kitz Professor of Anesthesia Research at Harvard Medical School, has suggested using capsaicin to deter abuse of certain extended-release drugs such as OxyContin and Ritalin. When taken as prescribed, opioid prescription drugs such as OxyContin or stimulant drugs such as Adderall XR release their active chemical over time, but when crushed and snorted, taken as a suppository, chewed, or injected, the larger than normal dosage is absorbed all at once and a much stronger effect is produced that can be highly addictive and dangerous due to the higher risk of overdose. Woolf has argued that adding capsaicin into the capsules would be a safe way to deter abuse. A person taking the capsule in the prescribed way (i.e., swallowing it whole) would suffer no ill effects from the additive. However, a person crushing it would expose the irritant. Anyone then swallowing it, snorting it, or injecting it would be exposed to the full power of the chemical. "Imagine snorting an extract of 50 jalapeño peppers and you get the idea," Woolf said in an interview with the Harvard University Gazette. As of 2006, Woolf's proposal is still in the preliminary stages of development and the additive has not yet entered the production stage. However, the two substances can be separated using cold water extraction.
Pest deterrent
Capsaicin is also used to deter pests. A common example is the use of ground-up or crushed dried chili pods in birdseed to deter squirrels, since birds are unaffected by capsaicin. Insects are also unaffected by capsaicin.
Mechanism of action
The burning and painful sensations associated with capsaicin result from its chemical interaction with sensory neurons. Capsaicin, as a member of the vanilloid family, binds to a receptor called the vanilloid receptor subtype 1 (VR1). First cloned in 1997, VR1 is an ion channel-type receptor. VR1, which can also be stimulated with heat and physical abrasion, permits cations to pass through the cell membrane and into the cell when activated. The resulting "depolarization" of the neuron stimulates it to signal the brain. By binding to the VR1 receptor, the capsaicin molecule produces the same effect that excessive heat or abrasive damage would cause, explaining why the spiciness of capsaicin is described as a burning sensation.
The VR1 ion channel has subsequently been shown to be a member of the superfamily of TRP ion channels, and as such is now referred to as TRPV1. There are a number of different TRP ion channels that have been shown to be sensitive to different ranges of temperature and probably are responsible for our range of temperature sensation. Thus, capsaicin does not actually cause a chemical burn; it causes only the sensation of one.
Capsaicin "high"
The "capsaicin high" is a euphoric sensation caused by the consumption of large quantities of capsaicin from capsaicin-laden foods. It is theorized that the pain induced by capsaicin causes the human body to release endorphins. Eventually, enough are released to create a sensation that is frequently compared to "runner's high".[1]
References
- Cromie WJ (2006) "Using chili peppers to burn drug abusers" Harvard University Gazette accessed 24 January 2006
- Dray A (1992) "Mechanism of action of capsaicin-like molecules on sensory neurons" Life Sci 51(23):1759-65
- Garnanez RJ, McKee LH (2001) "Temporal effectiveness of sugar solutions on mouth burn by capsaicin" IFT Annual Meeting 2001
- Henkin R (1991) '"Cooling the burn from hot peppers" JAMA 266(19):2766
- Nasrawi CW, Pangborn RM (1990) "Temporal effectiveness of mouth-rinsing on capsaicin mouth-burn" Physiol Behav 47(4):617-23
- Tewksbury JJ, Nabhan GP (2001) "Seed dispersal: Directed deterrence by capsaicin in chillies" Nature 412, 403-404 (26 July 2001), doi: 10.1038/35086653
See also
- Piperine, the active piquant chemical in black pepper
- Allyl isothiocyanate, the active piquant chemical in mustard, radishes, horseradish and wasabi
- Allicin, the active piquant flavor chemical in uncooked garlic and onions (see those articles for discussion of other chemicals in them relating to pungency, and eye irritation)
External links
- Capsaicin and Its Therapeutic Potential
- Molecule of the Month
- European Commission, opinion of the Scientific Committee on Food on capsaicin.
- An online article on popular capsaicin applications by Dave DeWitt
- A WikiHow article on How to Cool Chili Pepper Burns.
- The Straight Dope staff report: Are birds immune to hot pepper, enabling them to eat vast amounts and spread the seeds?

