Zinc chloride
| Zinc chloride | |
|---|---|

| |
| General | |
| Systematic name | Zinc chloride |
| Other names | Zinc(II) chloride, zinc dichloride, butter of zinc |
| Molecular formula | ZnCl2 |
| Molar mass | 136.29 g/mol |
| Appearance | White crystalline solid. |
| CAS number | [7646-85-7] |
| Properties | |
| Density and phase | 2.907 g/cm³, solid |
| Solubility in water | 432 g/100 mL (25 °C) |
| in ethanol | 100 g/100 mL (12.5 °C) |
| in acetone, diethyl ether |
Soluble |
| Melting point | 275 °C (548 K) |
| Boiling point | 756 °C (1029 K) |
| Structure | |
| Coordination geometry |
Tetrahedral, 4-coordinate, linear in the gas phase. |
| Crystal structure | Four forms known Hexagonal close-packed (δ) is the only stable form when anhydrous. |
| Dipole moment | ? D |
| Hazards | |
| MSDS | External MSDS |
| EU classification | Irritant (I), Corrosive(C). |
| NFPA 704 | |
| R-phrases | Template:R34, Template:R50, Template:R53 |
| S-phrases | Template:S7/8, Template:S28, Template:S45, Template:S60, Template:S61 |
| RTECS number | ZH1400000 |
| Supplementary data page | |
| Structure and properties |
n, εr, etc. |
| Thermodynamic data | Phase behaviour Solid, liquid, gas |
| Spectral data | UV, IR, NMR, MS |
| Related compounds | |
| Other anions | Zinc fluoride, zinc bromide, zinc iodide |
| Other cations | Copper(II) chloride, cadmium chloride |
| Except where noted otherwise, data are given for materials in their standard state (at 25°C, 100 kPa) Infobox disclaimer and references | |
Zinc chloride is the name of chemical compound ZnCl2 or its hydrates. All zinc chlorides are colorless or white and highly soluble in water. ZnCl2 itself is hygroscopic and very deliquescent. Samples should therefore be protected from sources of moisture, including the water vapor present in ambient air.
Four crystalline forms of ZnCl2 and its hydrates are known, although the anhydrous form appears to exist only in the hexagonal close-packed phase. Rapid cooling of molten ZnCl2 forms a glassy material.
Concentrated aqueous solutions of zinc chloride have the interesting property of dissolving starch, silk, and cellulose. Thus, solutions of zinc chlorides cannot be filtered through standard filter papers.
Zinc chloride finds wide application in textile processing, metallurgical fluxes and chemical synthesis.
Chemical properties
ZnCl2 is an ionic solid, although some covalent character is indicated by its low melting point (275 °C). Further evidence for covalency is provided by its high solubility in solvents such as diethyl ether. ZnCl2 is a mild Lewis acid. Consistent with this character, aqueous solutions of ZnCl2 have a pH around 4. It is hydrolyzed to an oxychloride when hydrated forms are heated.
In aqueous solution, zinc chloride is a useful source of Zn2+ for the preparation of other zinc salts, for example zinc carbonate:
Preparation and purification
Anhydrous ZnCl2 can be prepared from zinc and hydrogen chloride.
- Zn + 2 HCl → ZnCl2 + H2
Hydrated forms and aqueous solutions may be readily prepared using concentrated hydrochloric acid and pieces of Zn. Zinc oxide and zinc sulfide react with HCl, without forming hydrogen:
Commercial samples of zinc chloride typically contain water and zinc oxychloride, the main hydrolysis product. Such samples may be purified by refluxing the crude material with zinc powder in dioxane, after which the hot solution is filtered and, upon cooling, pure ZnCl2 precipitates. Anhydrous samples can be purified by sublimation in a stream of hydrogen chloride gas, followed by heating to 400 °C in a stream of dry nitrogen gas.
Uses
ZnCl2 is used as a flux for soldering because of its ability (when molten) to dissolve metal oxides. Typically this flux was prepared by dissolving zinc foil in dilute hydrochloric acid until the liquid ceased to evolve hydrogen, for this reason such flux was known as killed spirits. because of its corrosive nature it is not a suitable flux for situations where any residue cannot be cleaned totally away, such as electronic work. This property also leads to its use in the manufacture of magnesia cements for dental fillings and certain mouthwashes as an active ingredient. ZnCl2 has also been used as a fireproofing agent, for etching metals, and is also a primary ingredient in fabric refresheners such as Febreze.
In the laboratory, zinc chloride finds wide use, principally as a moderate-strength Lewis acid. It can catalyse (A) the Fischer indole synthesis[1], and also (B) Friedel-Crafts acylation reactions involving activated aromatic rings[2][3]
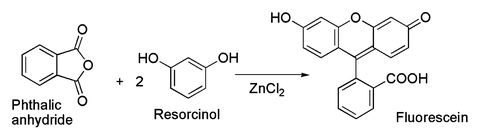
Related to the latter is the classical preparation of the dye fluorescein from phthalic anhydride and resorcinol, which involves a Friedel-Crafts acylation.[4] This transformation has in fact been accomplished using even the wet ZnCl2 sample shown in the picture above.
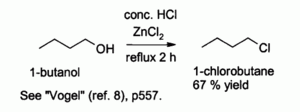
Hydrochloric acid alone reacts poorly with primary alcohols and secondary alcohols, but a combination of HCl with ZnCl2 (known together as the "Lucas reagent") is effective for the preparation of alkyl chlorides. Typical reactions are conducted at 130 °C. This reaction probably proceeds via an SN2 mechanism with primary alcohols but SN1 pathway with secondary alcohols.
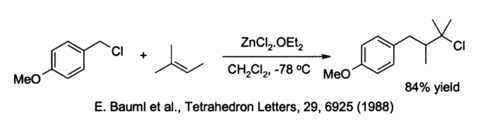
Zinc chloride also activates benzylic and allylic halides towards substitution by weak nucleophiles such as alkenes[5]:
In similar fashion, ZnCl2 promotes selective NaBH3CN reduction of tertiary, allylic or benzylic halides to the corresponding hydrocarbons.
Zinc chloride is also a useful starting reagent for the synthesis of many organozinc reagents, such as those used in the palladium catalysed Negishi coupling with aryl halides or vinyl halides.[6] In such cases the organozinc compound is usually prepared by transmetallation from an organolithium or a Grignard reagent, for example:
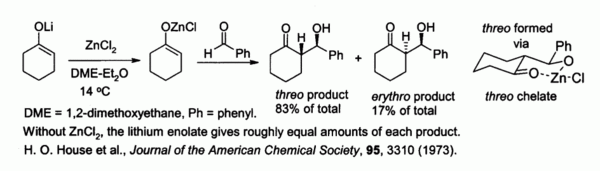
Zinc enolates, prepared from alkali metal enolates and ZnCl2, provide control of stereochemistry in aldol condensation reactions due to chelation on to the zinc. In the example shown below, the threo product was favored over the erythro by a factor of 5:1 when ZnCl2 in DME/ether was used.[7] The chelate is more stable when the bulky phenyl group is pseudo-equatorial rather than pseudo-axial, i.e., threo rather than erythro.
Precautions
Corrosive, irritant. Wear gloves and goggles.
References
- ^ R. L. Shriner, W. C. Ashley, E. Welch, in Organic Syntheses Collective Volume 3, p 725, Wiley, New York, 1955.
- ^ S. R. Cooper, in Organic Syntheses Collective Volume 3, p 761, Wiley, New York, 1955.
- ^ S. Y. Dike, J. R. Merchant, N. Y. Sapre, Tetrahedron, 47, 4775 (1991)
- ^ B. S. Furnell et al., Vogel's Textbook of Practical Organic Chemistry, 5th edition, Longman/Wiley, New York, 1989.
- ^ E. Bauml, K. Tschemschlok, R. Pock, H. Mayr, Tetrahedron Letters, 29, 6925 (1988)
- ^ S. Kim, Y. J. Kim, K. H. Ahn, Tetrahedron Letters, 24, 3369 (1983).
- ^ H. O. House, D. S. Crumrine, A. Y. Teranishi, H. D. Olmstead, Journal of the American Chemical Society, 95, 3310 (1973)
Bibliography
- N. N. Greenwood, A. Earnshaw, Chemistry of the Elements, 2nd ed., Butterworth-Heinemann, Oxford, UK, 1997.
- Handbook of Chemistry and Physics, 71st edition, CRC Press, Ann Arbor, Michigan, 1990.
- The Merck Index, 7th edition, Merck & Co, Rahway, New Jersey, USA, 1960.
- D. Nicholls, Complexes and First-Row Transition Elements, Macmillan Press, London, 1973.
- A. F. Wells, 'Structural Inorganic Chemistry, 5th ed., Oxford University Press, Oxford, UK, 1984.
- J. March, Advanced Organic Chemistry, 4th ed., p. 723, Wiley, New York, 1992.
- G. J. McGarvey, in Handbook of Reagents for Organic Synthesis, Volume 1: Reagents, Auxiliaries and Catalysts for C-C Bond Formation, (R. M. Coates, S. E. Denmark, eds.), pp. 220-3, Wiley, New York, 1999.