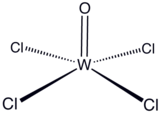
Tungsten(VI) oxytetrachloride
Appearance
| |

| |
| Names | |
|---|---|
| Other names
Tungsten(IV) chloride oxide
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.033.497 |
| EC Number |
|
PubChem CID
|
|
| |
| |
| Properties | |
| WOCl4 | |
| Molar mass | 341.651 g/mol |
| Appearance | red crystals |
| Density | 11.92 g/cm3 |
| Melting point | 211 °C (412 °F; 484 K) |
| Boiling point | 227.55 °C (441.59 °F; 500.70 K) |
| reacts | |
| Solubility | soluble in benzene and CS2 |
| Hazards | |
| GHS labelling:[1] | |

| |
| Warning | |
| H315, H319, H335 | |
| P261, P264, P271, P280, P302+P352, P304+P340, P305+P351+P338, P312, P321, P332+P313, P337+P313, P362, P403+P233, P405, P501 | |
| Related compounds | |
Other anions
|
Tungsten(VI) oxytetrafluoride Tungsten(VI) oxytetrabromide |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Tungsten(VI) oxytetrachloride is the inorganic compound with the formula WOCl4. This diamagnetic solid is used to prepare other complexes of tungsten. The red crystalline compound is soluble in nonpolar solvents but it reacts with alcohols and water and forms adducts with Lewis bases.[citation needed]
Structure
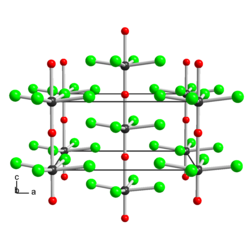
The solid consists of weakly associated square pyramidal monomers.[2] The compound is classified as an oxyhalide.
Synthesis and reactions
WOCl4 is prepared from tungsten trioxide:[3]
It is "difficult to prepare by other means."[4]
WOCl4 is Lewis acidic. It is a precursor to catalysts used for polymerization of alkynes.[5]
References
- ^ "Tungsten tetrachloride oxide". pubchem.ncbi.nlm.nih.gov. Retrieved 12 December 2021.
- ^ Hess, H.; Hartung, H. (1966). "Die Kristallstruktur von Wolframoxidchlorid WOCl4 und Wolframoxidbromid WOBr4". Z. Anorg. Allg. Chem. 34 (3–4): 157–166. doi:10.1002/zaac.19663440306.
- ^ Nielson, A. J. (2007). "Tungsten and Molybdenum Tetrachloride Oxides". Inorg. Synth. 23: 195–198. doi:10.1002/9780470132548.ch41. ISBN 9780470132548.
- ^ Audrieth, Ludwig F.; Kleinberg, Jacob (1953). Non-aqueous solvents. New York: John Wiley & Sons. p. 224. LCCN 52-12057.
- ^ Hayano, S.; Masuda, T. (1999). "Living Polymerization of [o-(Trifluoromethyl)phenyl]acetylene by WOCl4-Based Catalysts such as WOCl4-n-Bu4Sn-t-BuOH (1:1:1)". Macromolecules. 32: 7344–7348. doi:10.1002/zaac.19663440306.
