Coral
| Coral | |
|---|---|

| |
| Pillar coral, Dendrogyra cylindricus | |
| Scientific classification | |
| Kingdom: | |
| Phylum: | |
| Class: | Ehrenberg, 1831
|
| Extant Subclasses and Orders | |
|
Alcyonaria | |
Corals are marine organisms from the class Anthozoa and exist as small sea anemone-like polyps, typically in colonies of many identical individuals. The group includes the important reef builders that are found in tropical oceans, which secrete calcium carbonate to form a hard skeleton.
A coral "head", commonly perceived to be a single organism, is formed from myriads of individual but genetically identical polyps, each polyp only a few millimeters in diameter. Over thousands of generations, the polyps lay down a skeleton that is characteristic of their species. An individual head of coral grows by asexual reproduction of the individual polyps. Corals also breed sexually by spawning, with corals of the same species releasing gametes simultaneously over a period of one to several nights around a full moon.
Although corals can catch small fish and animals such as plankton using stinging cells on their tentacles, these animals obtain most of their nutrients from photosynthetic unicellular algae called zooxanthellae. Consequently, most corals depend on sunlight and grow in clear and shallow water, typically at depths shallower than 60 m (200 ft). These corals can be major contributors to the physical structure of the coral reefs that develop in tropical and subtropical waters, such as the enormous Great Barrier Reef off the coast of Queensland, Australia. Other corals do not have associated algae and can live in much deeper water, with the cold-water genus Lophelia surviving as deep as 3000 m.[3] Examples of these can be found living on the Darwin Mounds located north-west of Cape Wrath, Scotland. Corals have also been found off the coast of Washington State and the Aleutian Islands in Alaska.
Corals coordinate behaviour by communicating with each other.[4]
Phylogeny

Corals belong to the class Anthozoa and are divided into two subclasses, depending on the number of tentacles or lines of symmetry, and a series of orders corresponding to their exoskeleton, nematocyst type and mitochondrial genetic analysis.[1][2][5] Those with eight tentacles are called octocorallia or Alcyonaria and comprise soft corals, sea fans and sea pens. Those with more than eight in a multiple of six are called hexacorallia or Zoantharia. This group includes reef-building corals (Scleractinians), sea anemones and zoanthids.
Anatomy

While a coral head appears to be a single organism, it is actually a head of many individual, yet genetically identical, polyps. The polyps are multicellular organisms that feed on a variety of small organisms, from microscopic plankton to small fish.
Polyps are usually a few millimeters in diameter, and are formed by a layer of outer epithelium and inner jellylike tissue known as the mesoglea. They are radially symmetrical with tentacles surrounding a central mouth, the only opening to the stomach or coelenteron, through which both food is ingested and waste expelled.
The stomach closes at the base of the polyp, where the epithelium produces an exoskeleton called the basal plate or calicle (L. small cup). This is formed by a thickened calciferous ring (annular thickening) with six supporting radial ridges (as shown below). These structures grow vertically and project into the base of the polyp. When polyps are physically stressed, they contract into the calyx so that virtually no part is exposed above the skeletal platform. This protects the organism from predators and the elements (Barnes, R.D., 1987; Sumich, 1996).[6][7]
The polyp grows by extension of vertical calices which are occasionally septated to form a new, higher, basal plate. Over many generations this extension forms the large calciferous (Calcium containing) structures of corals and ultimately coral reefs.
Formation of the calciferous exoskeleton involves deposition of the mineral aragonite by the polyps from calcium ions they acquire from seawater. The rate of deposition, while varying greatly across species and environmental conditions, can be as much as 10 g / m² of polyp / day (0.3 ounce / sq yd / day). This is light dependent, with night-time production 90% lower than that during the middle of the day.[8]

The polyp's tentacles trap prey using stinging cells called nematocysts. These are cells modified to capture and immobilize prey, such as plankton, by injecting poisons, firing very rapidly in response to contact. These poisons are usually weak but in fire corals are potent enough to harm humans. Nematocysts can also be found in jellyfish and sea anemones. The toxins injected by nematocysts immobilize or kill prey, which can then be drawn into the polyp's stomach by the tentacles through a contractile band of epithelium called the pharynx.
The polyps interconnect by a complex and well developed system of gastrovascular canals allowing significant sharing of nutrients and symbiotes. In soft corals these range in size from 50-500 μm in diameter and to allow transport of both metabolites and cellular components.[9]

Aside from feeding on plankton, many corals as well as other cnidarian groups such as sea anemones (e.g. Aiptasia), form a symbiotic relationship with a class of algae, zooxanthellae, of the genus Symbiodinium. The sea anemone Aiptasia, while considered a pest among coral reef aquarium hobbyists, has served as a valuable model organism in the scientific study of cnidarian-algal symbiosis. Typically a polyp harbors one particular species of algae. Via photosynthesis, these provide energy for the coral, and aid in calcification.[10] The algae benefit from a safe environment, and use the carbon dioxide and nitrogenous waste produced by the polyp. Due to the strain the algae can put on the polyp, stress on the coral often triggers ejection of the algae, known on a large scale as coral bleaching, as it is the algae that contribute to the brown coloration of corals; other colors, however, are due to host coral pigments, such as GFPs (green fluorescent protein). Ejecting the algae increases the polyps' chances of surviving stressful periods - they can regain the algae at a later time. If the stressful conditions persist, the corals eventually die.[11]
Reproduction
Corals can be both gonochoristic (unisexual) and hermaphroditic, each of which can reproduce sexually and asexually. Reproduction also allows coral to settle new areas.
Sexual
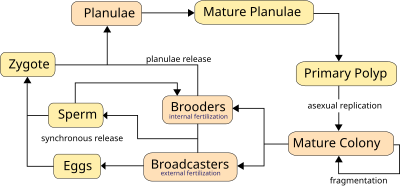
Corals predominantly reproduce sexually, with 25% of hermatypic corals (stony corals) forming single sex (gonochoristic) colonies, whilst the rest are hermaphroditic.[12] About 75% of all hermatypic corals "broadcast spawn" by releasing gametes - eggs and sperm - into the water to spread offspring over large distances. The gametes fuse during fertilisation to form a microscopic larvum called a planula, typically pink and elliptical in shape; a moderately sized coral colony can form several thousands of these larvae per year to overcome the huge odds against formation of a new colony.[13]
The planula swims towards light, exhibiting positive phototaxis, to surface waters where they drift and grow for a time before swimming back down to locate a surface on which it can attach and establish a new colony. At many stages of this process there are high failure rates, and even though millions of gametes are released by each colony very few new colonies form. The time from spawning to settling is usually 2 or 3 days, but can be up to 2 months.[14] The larva grows into a coral polyp and eventually becomes a coral head by asexual budding and growth, creating new polyps.

Corals that do not broadcast their eggs are called brooders, this is the case for most non-stony corals. These corals release sperm but harbour eggs, allowing larger, negatively buoyant, planulae to form which the polyp later releases ready to settle.[10] The larva grows into a coral polyp and eventually becomes a coral head by asexual budding.
Synchronous spawning is very typical on a coral reef and often, even when multiple species are present, all the corals on the reef release gametes the same night. This synchrony is essential so that male and female gametes can meet and form planula. The cues that guide the release are complex, but over the short term involve lunar changes, sunset time, and possibly chemical signalling.[12] Synchronous spawning may have the result of forming coral hybrids and is perhaps involved in coral speciation.[15] In some places the coral spawn can be dramatic, usually occurring at night, where the usually clear water becomes cloudy with gametes.
Corals must rely on environmental cues, varying from species to species, to determine the proper time to release gametes into the water. Corals use two methods for sexual reproduction, which differ in whether the female gametes are released:
- Broadcasters, the majority of which mass spawn, rely heavily on environmental cues, because in contrast to brooders they release both sperm and eggs into the water. The corals use long-term cues such as day length, water temperature, and/or rate of temperature change. The short-term cue is most often the lunar cycle, with the sunset cuing the time of release.[12] About 75% of coral species are broadcasters, the majority of which are hermatypic, or reef-building corals.[12] The positively buoyant gametes float towards the surface where fertilization occurs to produce planula larvae. The planula larvae swim towards the surface light to enter into currents, where they remain usually for two days, but can be up to three weeks, and in one known case two months,[14] after which they settle and metamorphose into polyps and form colonies.
- Brooders are most often ahermatypic (non-reef building) in areas of high current or wave action. Brooders release only sperm, which is negatively buoyant, and can store unfertilized eggs for weeks, lowering the need for mass synchronous spawning events, which do sometimes occur.[12] After fertilization the corals release planula larvae which are ready to settle.
Asexual

Within a coral head the genetically identical polyps reproduce asexually to allow colony growth. This is achieved either through gemmation (budding) or through division, both shown in the diagrams of Orbicella annularis. Budding involves a new polyp growing from an adult, whereas division forms two polyps each as large as the original.[13]
- Budding expands the size of a coral colony. It occurs when a new corallite grows out from the adult polyp. As the new polyp grows it produces a coelenteron (stomach), tentacles and a mouth. The distance between the new and adult polyps grows, and with it the coenosarc (the common body of the colony; see coral anatomy). Budding can occur by means of:
- Intra-tentacular budding forms from the oral discs of a polyp, meaning that both polyps are the same size and are within the same ring of tentacles.
- Extra-tentacular budding forms from the base of a polyp, and the new polyp is smaller.
- Longitudinal division begins with broadening of a polyp, which then divides the coelenteron. The mouth divides and new tentacles form. The two "new" polyps must generate their missing body parts and exoskeleton.
- Transversal division occurs when polyps and the exoskeleton divide transversally into two parts. This means that one has the basal disc (bottom) and the other has the oral disc (top). The two new polyps must again generate the missing parts.
- Fission occurs in some corals, especially among the family Fungiidae, where the colony is able to split into two or more colonies during the early stages of their development.
Whole colonies can reproduce asexually through fragmentation or bailout, forming another individual colony with the same genome.
- Polyp bailout occurs when a single polyp abandons the colony and re-establishes on a new substrate to create a new adult colony.
- Fragmentation, involves individuals broken from the colony during storms, or other situations where breaking can occur. The separated individuals can start new coral colonies.
Reefs

The hermatypic, stony corals are often found in coral reefs, large calcium carbonate structures generally found in shallow, tropical water. Reefs are built up from coral skeletons and held together by layers of calcium carbonate produced by coralline algae. Reefs are extremely diverse marine ecosystems being host to over 4,000 species of fish, massive numbers of cnidarians, mollusks, crustaceans, and many other animals.[16]
Types
Hermatypic corals
Hermatype corals or stony corals build reefs. With the help of zooxanthellae, they convert surplus food to calcium carbonate forming a hard skeleton. Hermatype-species include Scleractinia, Millepora, Tubipora and Heliopora. [17]
In the Caribbean alone 50 species of uniquely structured hard coral exist. Some of the most well known types being:
- Brain coral grow to 1.8 meters in width.
- Acropora and Staghorn coral grow fast and large and are important reef-builders. Staghorn coral displays large antler-like branches and grows in areas with strong surf.
- Galaxea fascicularis or star coral is another important reef-builder.
- Pillar coral forms pillars which can grow to 3 meters in height.
- Leptopsommia or rock coral, appears almost everywhere in the Caribbean[18].
Ahermatypic corals
Ahermatypic corals are corals that have no zooxanthellae and can therefore not build the solid skeletons that form reefs. They include Alcyonaceas, as well as some Anthipatharia-species (Black coral, Cirripathes, Antipathes). [17] Ahermatypic corals such as sea whips, sea feathers, and sea pens [18] are also known as soft corals. Unlike stony corals, they are flexible, moving back and forth in the current, and often are perforated, with a lace-like appearance. Their skeletons are made of protein, rather than calcium. Soft corals are somewhat less plentiful (in the Caribbean, twenty species appear) than stony corals.
Evolutionary history

Although corals first appeared in the Cambrian period,[19] some 542 million years ago, fossils are extremely rare until the Ordovician period, 100 million years later, when Rugose and Tabulate corals became widespread.
Tabulate corals occur in the limestones and calcareous shales of the Ordovician and Silurian periods, and often form low cushions or branching masses alongside Rugose corals. Their numbers began to decline during the middle of the Silurian period and they finally became extinct at the end of the Permian period, 250 million years ago. The skeletons of Tabulate corals are composed of a form of calcium carbonate known as calcite.
Rugose corals became dominant by the middle of the Silurian period, and became extinct early in the Triassic period. The Rugose corals existed in solitary and colonial forms, and are also composed of calcite.
The Scleractinian corals filled the niche vacated by the extinct Rugose and Tabulate species. Their fossils may be found in small numbers in rocks from the Triassic period, and become relatively common in the Jurassic and later periods. Scleractinian skeletons are composed of a form of calcium carbonate known as aragonite.[20] Although they are geologically younger than the Tabulate and Rugose corals, their aragonitic skeleton is less readily preserved, and their fossil record is less complete.
 | |
|
Timeline of the major coral fossil record and developments from 650 m.y.a. to present.[21][22] |
|
At certain times in the geological past corals were very abundant. Like modern corals, these ancestors built reefs, some of which now lie as great structures in sedimentary rocks.
Fossils of fellow reef-dwellers algae, sponges, and the remains of many echinoids, brachiopods, bivalves, gastropods, and trilobites appear along with coral fossils. This makes some corals useful index fossils, enabling geologists to date the age the rocks in which they are found.
Coral fossils are not restricted to reef remnants, and many solitary corals may be found elsewhere, such as Cyclocyathus, which occurs in England's Gault clay formation.
Environmental influences

Corals are highly sensitive to environmental changes. Scientists have predicted that over 50% of the world's coral reefs may be destroyed by the year 2030;[23] as a result most nations protect them through environmental laws. Algae can overwhelm a coral reef if too many nutrients are present. Coral will also die if the water temperature changes by more than a degree or two beyond its normal range or if the salinity of the water drops. In an early symptom of environmental stress, corals expel their zooxanthellae; without their symbiotic unicellular algae, coral tissues become colorless as they reveal the white of their calcium carbonate skeletons, an event known as coral bleaching.[24]
Many governments now prohibit removal of coral from reefs and use education to inform their populations about reef protection and ecology. However, many other human activities damage reefs, including mooring, fishing, diving, mining and construction.
The narrow niche that coral occupies, and the stony corals' reliance on calcium carbonate deposition, means they are susceptible to changes in water pH. The increase in atmospheric carbon dioxide has caused enough dissolution of carbon dioxide to lower the ocean's pH, in a process known as ocean acidification. Lowered pH reduces the ability of corals to produce calcium carbonate, and at the extreme, can entirely dissolve those skeletons. Without deep and immediate cuts in anthropogenic CO2 emissions, some scientists fear that ocean acidification will result in the severe degradation or destruction of coral species and ecosystems.[25]

Climatic variations can cause temperature changes that destroy corals. For example, during the 1997-98 warming event all the hydrozoan Millepora boschmai colonies near Panamá were bleached and died within six years - this species is now thought to be extinct.[26]
Uses
Live corals
Local economies near major coral reefs benefit from an abundance of fish and other marine creatures as a food source. Reefs also provide recreational scuba diving and snorkeling tourism. Unfortunately these activities can also have deleterious effects, such as accidental destruction of coral. Coral is also useful as a protection against hurricanes and other extreme weather.
Live coral is highly sought after in the aquarium trade. Provided the proper ecosystem, live coral makes a stunning addition to any salt water aquarium. Soft corals are considered easier to maintain in captivity than hard corals.[27]
Deep sea bamboo corals (Isididae) may be among the first organisms to display the effects of ocean acidification. They produce growth rings similar to those of tree and can provide a view of changes in the condition in the deep sea over time. [28] Other coral biology research presents the possibility that Isididae corals, because of their potential to mimic biological properties, may be usable as living bone implants and in aquatic cultivation.[29]
Coral as a gemstone
Intensely red coral is sometimes called fire coral (but this is not at all the same thing as fire coral). Red coral is very rare now because of overharvesting due to the great demand for perfect red coral in jewelry-making.
Ancient corals

Ancient coral reefs on land are often mined for lime or use as building blocks ("coral rag"). Coral rag is an important local building material in places such as the East African coast.
The annual growth bands in bamboo corals and others allow geologists to construct year-by-year chronologies, a form of incremental dating, which can provide high-resolution records of past climatic and environmental changes usinggeochemical techniques.[30]
Certain species of corals form communities called microatolls. The vertical growth of microatolls is limited by average tidal height. By analyzing the various growth morphologies, microatolls can be used as a low resolution record of patterns of sea level change. Fossilized microatolls can also be dated using radioactive carbon dating. Such methods have been used to used to reconstruct Holocene sea levels.[31]
See also
Gallery
Further images: commons:Category:Coral reefs and commons:Category:Coral
-
Fungia sp. skeleton
-
Brain coral, Diploria labyrinthiformis
-
Polyps of Eusmilia fastigiata
-
Staghorn coral, Acropora
-
Orange cup coral, Balanophyllia elegans
-
Brain coral spawning
-
Brain coral releasing eggs
References
- ^ a b
Daly, M., Fautin, D.G., and Cappola, V.A. (2003). "Systematics of the Hexacorallia (Cnidaria: Anthozoa)". Zoological Journal of the Linnean Society. 139: 419–437. doi:10.1046/j.1096-3642.2003.00084.x.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ a b
McFadden, C.S., France, S.C., Sanchez, J.A., and Alderslade, P. (2006). "A molecular phylogenetic analysis of the Octocorallia (Cnidaria: Anthozoa) based on mitochondrial protein-coding sequences". Molecular Phylogenentics and Evolution. 41 (3): 413–527. PMID 12967605.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^
Squires, D.F. (1959). [? "Deep sea corals collected by the Lamont Geological Observatory. 1. Atlantic corals"]. American Museum Novitates. 1965 (?): 1–42. ?.
{{cite journal}}: Check|url=value (help); Unknown parameter|month=ignored (help) - ^ Witzany G, Madl P. (2009). Biocommunication of corals. International Journal of Integrative Biology 5(3): 152-163.
- ^
France, S. C., P. E. Rosel, J. E. Agenbroad, L. S. Mullineaux, and T. D. Kocher (1996). "DNA sequence variation of mitochondrial large-subunit rRNA provides support for a two subclass organization of the Anthozoa (Cnidaria)". Molecular Marine Biology and Biotechnology. 5 (1): 15–28. PMID 8869515.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Barnes, R.D. (1987). Invertebrate Zoology; Fifth Edition. Orlando, FL, USA: Harcourt Brace Jovanovich, Inc. pp. 149–163.
- ^ Sumich, J. L. (1996). An Introduction to the Biology of Marine Life; Sixth Edition. Dubuque, IA, USA: Wm. C. Brown. pp. 255–269.
- ^ "Anatomy of Coral". Marine Reef. Retrieved 2006-03-31.
- ^
D. Gateno, A. Israel, Y. Barki and B. Rinkevich (1998). "Gastrovascular Circulation in an Octocoral: Evidence of Significant Transport of Coral and Symbiont Cells". The Biological Bulletin. 194 (2): 178–186. doi:10.2307/1543048.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b
Madl, P. and Yip, M. (2000). "Field Excursion to Milne Bay Province - Papua New Guinea". Retrieved 2006-03-31.
{{cite web}}: Cite has empty unknown parameter:|accessyear=(help)CS1 maint: multiple names: authors list (link) - ^ W. W. Toller, R. Rowan and N. Knowlton (2001). "Repopulation of Zooxanthellae in the Caribbean Corals Montastraea annularis and M. faveolata following Experimental and Disease-Associated Bleaching". The Biological Bulletin. 201: 360–373. doi:10.2307/1543614. PMID 11751248.
- ^ a b c d e Veron, J.E.N. (2000). Corals of the World. Vol 3 (3rd ed.). Australia: Australian Institute of Marine Sciences and CRR Qld Pty Ltd. ISBN 0-64232-236-8.
- ^ a b Barnes, R. and R. Hughes (1999). An Introduction to Marine Ecology (3rd ed.). Malden, MA: Blackwell Science, Inc. pp. 117–141. ISBN 0-86542-834-4.
- ^ a b Jones, O.A. and R. Endean. (1973). Biology and Geology of Coral Reefs. New York, USA: Harcourt Brace Jovanovich. pp. 205–245. ISBN 0-12-389602-9.
- ^
Hatta, M., Fukami, H., Wang, W., Omori, M., Shimoike, K., Hayashibara, T., Ina, Y., Sugiyama, T. (1999). "Reproductive and genetic evidence for a reticulate evolutionary theory of mass spawning corals". Molecular Biology and Evolution. 16 (11): 1607–1613. PMID 8096089.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Spalding, Mark, Corinna Ravilious, and Edmund Green (2001). World Atlas of Coral Reefs. Berkeley, CA, USA: University of California Press and UNEP/WCMC. pp. 205–245.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ a b The Greenpeace Book of Coral Reefs
- ^ a b National Geographic Traveller:The Caribbean
- ^
Pratt, B.R. (2001). "12: Ecology and Evolution of Cambrian Reefs". Ecology of the Cambrian Radiation. Columbia University Press. p. 259. ISBN 0231106130.
{{cite book}}:|access-date=requires|url=(help); External link in|chapterurl=|chapterurl=ignored (|chapter-url=suggested) (help); Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^
Ries, J.B., Stanley, S.M., Hardie, L.A. (2006). "Scleractinian corals produce calcite, and grow more slowly, in artificial Cretaceous seawater". Geology. 34: 525–528. doi:10.1130/G22600.1. 10.1130/G22600.1.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Waggoner, Ben M. (2000). Smith, David; Collins, Allen (eds.). "Anthozoa: Fossil Record". Anthozoa. UCMP. Retrieved 9 March 2020.
- ^ Oliver, William A. Jr. (2003). "Corals: Table 1". Fossil Groups. USGS. Archived from the original on 9 January 2009. Retrieved 9 March 2020.
- ^ Norlander (8 December 2003). "Coral crisis! Humans are killing off these bustling underwater cities. Can coral reefs be saved? (Life science: corals)". Science World.
- ^ Hoegh-Guldberg, O. (1999). "Climate change, coral bleaching and the future of the world's coral reefs" (PDF). Marine and Freshwater Research. 50 (8): 839–866. doi:10.1071/MF99078.
- ^ Gattuso, J.P., Frankignoulle, M., Bourge, I., Romaine, S. and Buddemeier, R.W. (1998). "Effect of calcium carbonate saturation of seawater on coral calcification". Global Planet Change. 18: 37–46. doi:10.1016/S0921-8181(98)00035-6.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^
Glynn, P.W. (2001). "History of significant coral bleaching events and insights regarding amelioration". Coral Bleaching and Marine Protected Areas: Proceedings of the Workshop on Mitigating Coral Bleaching Impact Through MPA Design. Bishop Museum, Honolulu, Hawaii, 29-31 May 2001: 36–39.
{{cite journal}}:|format=requires|url=(help); Cite has empty unknown parameter:|month=(help) - ^ "Eight great soft corals for new reefkeepers". AquaDaily. 2008-12-05. Retrieved 2009-01-02.
- ^ "National Oceanic and Atmospheric Administration - New Deep-Sea Coral Discovered on NOAA-Supported Mission". www.noaanews.noaa.gov. Retrieved 2009-05-11.
- ^
H. Ehrlich, P. Etnoyer, S. D. Litvinov; et al. "Biomaterial structure in deep-sea bamboo coral (Anthozoa: Gorgonacea: Isididae)". www3.interscience.wiley.com. doi:0.1002/mawe.200600036. Retrieved 2009-05-11.
{{cite web}}: Check|doi=value (help); Explicit use of et al. in:|first=(help)CS1 maint: multiple names: authors list (link) - ^
Schrag, D.P. and Linsley, B.K. (2002). "Corals, Chemistry, and Climate". Science. 296 (8): 277–278. doi:10.1126/science.1071561. PMID 11951026.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^
Smithers, S.G. and Woodroffe, C.D. (2000). "Microatolls as sea-level indicators on a mid-ocean atoll". Marine Geology. 168 (1–4): 61–78. doi:10.1016/S0025-3227(00)00043-8.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link)
Further reading
- Coral, The Reef & Marine Aquarium Magazine. ISSN 1556-5769 Coral Magazine
- Book of Coral Propagation by Anthony Calfo. ISBN 0980236509
- Coral Reefs of the World by Susan Wells
- Corals of the World: Biology and Field Guide by Surrey Redhill
- Marine Biology, An Ecological Approach, Sixth Edition by Nybakken, J.W. 2004. ISBN 0805345825
- Indo-Pacific Coral Reef Field Guide by Allen, G.R & R. Steene. 1994. ISBN 9810056877
- Coral Reef Animals of the Indo-Pacific, Animals Life from Africa to Hawai‘i (invertebrates) by Gosliner, T., D. Behrens & G. Williams. 1996. ISBN 0930118219
- Tropical Pacific Invertebrates by Colin, P.L. & C. Arneson. 1995. ISBN 0964562502
- Corals of Australia and the Indo-Pacific by Veron, J.E.N. 1993. ISBN 0824815041
- The Evolution of Reef Communities by Fagerstrom, J.A. 1987. ISBN 0471815284
- A Reef Comes to Life. Creating an Undersea Exhibit by Segaloff, Nat, and Paul Erickson. 1991. ISBN 0531109941
- SeaWorld - Coral reef bibliography
External links
- Precht, William F. Coral Reef Restoration Handbook ISBN 0849320739
- Microdocs: What is a coral?
- NOAA CoRIS - Coral Reef Biology
- NOAA Ocean Service Education - Corals
- University of Southern Mississippi - Coral Reef Resource Guide








