Synthetic diamond
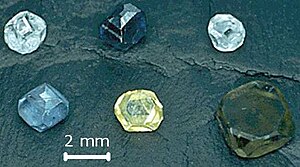
Synthetic diamond is diamond produced in a technological process, as opposed to natural diamond which is created in geological processes. Synthetic diamond is also widely known as HPHT diamond or CVD diamond where HPHT and CVD refer to the production method, namely high-pressure high-temperature synthesis and chemical vapor deposition, respectively.
Numerous claims of diamond synthesis were documented between 1879 and 1928; each of those attempts were carefully analyzed and none were confirmed. In the 1940s, systematic research began in the United States, Sweden and the Soviet Union to grow diamond using CVD and HPHT processes. The first reproducible synthesis was reported around 1953. Those two processes still dominate the production of synthetic diamond. A third method, known as detonation synthesis, has entered the diamond market in the late 1990s. In this process, nanometer-sized diamond grains are created in a detonation of carbon-containing explosives. A fourth method, treating graphite with high-power ultrasound, has been demonstrated in the laboratory, but as yet there is no commercial application.
The properties of synthetic diamond depend on the details of the manufacturing processes, and can be inferior or superior to those of natural diamond; the hardness, thermal conductivity and electron mobility are superior in some synthetic diamonds (either HPHT or CVD). Consequently, synthetic diamond is widely used in abrasives, in cutting and polishing tools and in heat sinks. Electronic applications of synthetic diamond are being developed, including high-power switches at power stations, high-frequency field-effect transistors and light-emitting diodes. Synthetic diamond detectors of ultraviolet (UV) light or high-energy particles are used at high-energy research facilities and are available commercially. Because of its unique combination of thermal and chemical stability, low thermal expansion and high optical transparency in a wide spectral range, synthetic diamond is becoming the most popular material for optical windows in high-power CO2 lasers and gyrotrons.
Both CVD and HPHT diamonds can be cut into gems and various colors can be produced: clear white, yellow, brown, blue, green and orange. The appearance of synthetic gems on the market created major concerns in the diamond trading business, as a result of which special spectroscopic devices and techniques have been developed to distinguish synthetic and natural diamonds.
History
After the 1797 discovery that diamond was pure carbon, many attempts were made to convert various cheap forms of carbon into diamond. The earliest successes were reported by James Ballantyne Hannay in 1879[1] and by Ferdinand Frédéric Henri Moissan in 1893. Their method involved heating charcoal at up to 3500 °C with iron inside a carbon crucible in a furnace. Whereas Hannay used a flame-heated tube, Moissan applied his newly developed electric arc furnace, in which an electric arc was struck between carbon rods inside blocks of lime.[2] The molten iron was then rapidly cooled by immersion in water. The contraction generated by the cooling supposedly produced the high pressure required to transform graphite into diamond. Moissan published his work in a series of articles in the 1890s.[3]
Many other scientists tried to replicate his experiments. Sir William Crookes claimed success in 1909. Otto Ruff claimed in 1917 to have produced diamonds up to 7 mm in diameter,[4] but later retracted his statement.[5] In 1926, Dr. Willard Hershey of McPherson College replicated Moissan's and Ruff's experiments,[6][7] producing a synthetic diamond; that specimen is on display at the McPherson Museum in Kansas.[8] Despite the claims of Moissan, Ruff, and Hershey, other experimenters were unable to reproduce their synthesis.[9][10]
The most definitive replication attempts were performed by Sir Charles Algernon Parsons. A prominent scientist and engineer known for his invention of the steam turbine, he spent 30 years (1882–1922) and a considerable part of his fortune trying to reproduce the experiments of Moissan and Hannay, but also adapted processes of his own. Parsons was known for his painstakingly accurate approach and methodical record keeping; all his resulting samples were preserved for further analysis by an independent party.[11] He wrote a number of articles—some of the earliest on HPHT diamond—in which he claimed to have produced small diamonds.[12] However in 1928 he authorized Dr. C.H. Desch to publish an article[13] in which he stated his belief that no synthetic diamonds (including those of Moissan and others) had been produced up to that date. He suggested that most diamonds that had been produced up to that point were likely synthetic spinel.[9]
GE diamond project
In 1941, an agreement was made between the General Electric (GE), Norton and Carborundum companies to further develop diamond synthesis. They were able to heat carbon to about 3000 °C under a pressure of 3.5 gigapascals (GPa) for a few seconds. Soon thereafter the Second World War interrupted the project. It was resumed in 1951 at the Schenectady Laboratories of GE, and a high-pressure diamond group was formed with F.P. Bundy and H.M. Strong. Tracy Hall and others joined this project shortly thereafter.[14]
The Schenectady group improved on the anvils designed by Percy Bridgman, who received a Nobel Prize for his work in 1946. Bundy and Strong made the first improvements, then more were made by Hall. The GE team used tungsten carbide anvils within a hydraulic press to squeeze the carbonaceous sample held in a catlinite container, the finished grit being squeezed out of the container into a gasket. The team recorded diamond synthesis on one occasion, but the experiment could not be reproduced because of uncertain synthesis conditions.[15]

Hall achieved the first commercially successful synthesis of diamond on December 16, 1954, and this was announced on February 15, 1955. His breakthrough was using a "belt" press, which was capable of producing pressures above 10 GPa and temperatures above 2000 °C.[16] The "belt" press (see below) used a pyrophyllite container in which graphite was dissolved within molten nickel, cobalt or iron. Those metals acted as a "solvent-catalyst", which both dissolved carbon and accelerated its conversion into diamond. The largest diamond he produced was 0.15 mm across; it was too small and visually imperfect for jewelry, but usable in industrial abrasives. Hall's co-workers were able to replicate his work, and the discovery was published in the major journal Nature.[17][18] He was the first person to grow a synthetic diamond with a reproducible, verifiable and well-documented process. He left GE in 1955, and three years later developed a new apparatus for the synthesis of diamond—a tetrahedral press with four anvils—to avoid infringing his previous patent, which was still assigned to GE.[19] Hall received the American Chemical Society Award for Creative Invention for his work in diamond synthesis.[20]
Later developments
An independent diamond synthesis was achieved on February 16, 1953 in Stockholm, Sweden by the ASEA (Allmänna Svenska Elektriska Aktiebolaget), one of Sweden's major electrical manufacturing companies. Starting in 1949, ASEA employed a team of five scientists and engineers as part of a top-secret diamond-making project code-named QUINTUS. The team used a bulky split-sphere apparatus designed by Baltzar von Platen and Anders Kämpe.[14][21] Pressure was maintained within the device at an estimated 8.4 GPa for an hour. A few small diamonds were produced, but not of gem quality or size. The work was not reported until the 1980s.[22] During the 1980s, a new competitor emerged in Korea, a company named Iljin Diamond; it was followed by hundreds of Chinese enterprises. Iljin Diamond allegedly accomplished diamond synthesis in 1988 by misappropriating trade secrets from GE via a Korean former GE employee.[23][24]

Synthetic gem-quality diamond crystals were first produced in 1970 by GE, then reported in 1971. The first successes used a pyrophyllite tube seeded at each end with thin pieces of diamond. The graphite feed material was placed in the center and the metal solvent (nickel) between the graphite and the seeds. The container was heated and the pressure was raised to about 5.5 GPa. The crystals grow as they flow from the center to the ends of the tube, and extending the length of the process produces larger crystals. Initially a week-long growth process produced gem-quality stones of around 5 mm (1 carat or 0.2 g), and the process conditions had to be as stable as possible. The graphite feed was soon replaced by diamond grit because that allowed much better control of the shape of the final crystal.[18]
The first gem-quality stones were always yellow to brown in color because of contamination with nitrogen. Inclusions were common, especially "plate-like" ones from the nickel. Removing all nitrogen from the process by adding aluminum or titanium produced colorless "white" stones, and removing the nitrogen and adding boron produced blue ones.[25] However, removing nitrogen slowed the growth process and reduced the crystalline quality, so the process was normally run with nitrogen present.
Although the GE stones and natural diamonds were chemically identical, their physical properties were not the same. The colorless stones produced strong fluorescence and phosphorescence under short-wavelength ultraviolet light, but were inert under long-wave UV. Among natural diamonds, only the rarer blue gems exhibit these properties. Contrary to natural diamonds, all the GE stones showed strong yellow fluorescence under X-rays.[26] The De Beers Diamond Research Laboratory has grown stones of up to 25 carats (5.0 g) for research purposes. Stable HPHT conditions were kept for six weeks to grow high-quality diamonds of this size. For economic reasons, the growth of most synthetic diamonds is terminated when they reach a weight of 1 carat (200 mg) to 1.5 carats (300 mg).[27]
In the 1950s, research started in the Soviet Union and the US on the growth of diamond by pyrolysis of hydrocarbon gases at the relatively low temperature of 800 °C. This low-pressure process is known as chemical vapor deposition (CVD). William G. Eversole reportedly achieved vapor deposition of diamond over diamond substrate in 1953, but it was not reported until 1962.[28] Diamond film deposition was independently reproduced by Angus and coworkers in 1968[29] and by Deryagin and Fedoseev in 1970.[30] Whereas Eversole and Angus used large, expensive, single-crystal diamonds as substrates, Deryagin and Fedoseev succeeded in making diamond films on non-diamond materials (silicon and metals), which led to massive research on inexpensive diamond coatings in the 1980s.[31]
Manufacturing technologies
There are several methods used to produce synthetic diamond. The original method uses high pressure and high temperature (HPHT) and is still widely used because of its relatively low cost. The process involves large presses that can weigh hundreds of tons to produce a pressure of 5 GPa at 1500 °C. The second method, using chemical vapor deposition (CVD), creates a carbon plasma over a substrate onto which the carbon atoms deposit to form diamond. Other methods include explosive formation (forming detonation nanodiamonds) and sonication of graphite solutions.[32][33][34]
High pressure, high temperature
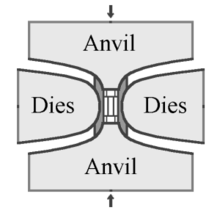
In the HPHT method, there are three main press designs used to supply the pressure and temperature necessary to produce synthetic diamond: the belt press, the cubic press and the split-sphere (BARS) press.
The original GE invention by Tracy Hall uses the belt press wherein the upper and lower anvils supply the pressure load to a cylindrical inner cell. This internal pressure is confined radially by a belt of pre-stressed steel bands. The anvils also serve as electrodes providing electrical current to the compressed cell. A variation of the belt press uses hydraulic pressure, rather than steel belts, to confine the internal pressure.[35] Belt presses are still used today, but they are built on a much larger scale than those of the original design.[36]
The second type of press design is the cubic press. A cubic press has six anvils which provide pressure simultaneously onto all faces of a cube-shaped volume.[37] The first multi-anvil press design was actually a tetrahedral press, using only four anvils to converge upon a tetrahedron-shaped volume.[38] The cubic press was created shortly thereafter to increase the volume to which pressure could be applied. A cubic press is typically smaller than a belt press and can more rapidly achieve the pressure and temperature necessary to create synthetic diamond. However, cubic presses cannot be easily scaled up to larger volumes: the pressurized volume can be increased by using larger anvils, but this also increases the amount of force needed on the anvils to achieve the same pressure. An alternative is to decrease the surface area to volume ratio of the pressurized volume, by using more anvils to converge upon a higher-order platonic solid, such as a dodecahedron. However, such a press would be unnecessarily complex and difficult to manufacture.[37]

BARS is the most compact, efficient, and economical of all the diamond-producing presses. In the center of a BARS device, there is a ceramic cylindrical "synthesis capsule" of about 2 cm3 in size. The cell is placed into a cube of pressure-transmitting material, such as pyrophyllite ceramics, which is pressed by inner anvils made from cemented carbide (e.g., tungsten carbide or VK10 hard alloy).[39] The outer octahedral cavity is pressed by 8 steel outer anvils. After mounting, the whole assembly is locked in a disc-type barrel with a diameter about 1 meter. The barrel is filled with oil, which pressurizes upon heating, and the oil pressure is transferred to the central cell. The synthesis capsule is heated up by a coaxial graphite heater and the temperature is measured with a thermocouple.[40]
Chemical vapor deposition
Chemical vapor deposition of diamond is a method of growing diamond from a hydrocarbon gas mixture. Since the early 1980s, this method has been the subject of intensive worldwide research. Whereas the mass-production of high-quality diamond crystals make the HPHT process the more suitable choice for industrial applications, the flexibility and simplicity of CVD setups explain the popularity of CVD growth in laboratory research. The advantages of CVD diamond growth include the ability to grow diamond over large areas and on various substrates, and the fine control over the chemical impurities and thus properties of the diamond produced. Unlike HPHT, CVD process does not require high pressures, as the growth typically occurs at pressures under 27 kPa.[32][41]
The CVD growth involves substrate preparation, feeding varying amounts of gases into a chamber and energizing them. The substrate preparation includes choosing an appropriate material and its crystallographic orientation; cleaning it, often with a diamond powder to abrade a non-diamond substrate; and optimizing the substrate temperature (about 800 °C) during the growth through a series of test runs. The gases always include a carbon source, typically methane, and hydrogen with a typical ratio of 1:99. Hydrogen is essential because it selectively etches off non-diamond carbon. The gases are ionized into chemically active radicals in the growth chamber using microwave power, a hot filament, an arc discharge, a welding torch, a laser, an electron beam, or other means.
One of the important factors in chamber design is that during the growth, the chamber materials are etched off by the plasma and can incorporate into the growing diamond. In particular, CVD diamond is often contaminated by silicon originating from the silica windows of the growth chamber or from the silicon substrate.[42] Therefore, silica windows are either avoided or moved away from the substrate. Also, once boron-containing species are introduced into the chamber, it becomes unsuitable for growth of pure diamond.[32][41]
CVD and the Ig Nobel Prize's Chemistry Prize, 2009
The Ig Nobel Prize for Chemistry was won in 2009 by three Mexican researchers Javier Morales, Miguel Apatiga and Victor M. Castano, for research published in 2008 showing that thin films of synthetic diamond can be produced from tequila using a process of Pulsed Liquid Injection Chemical Vapor Deposition (PLI-CVD) onto both silicon (100) and stainless steel 304 at 850 °C (1,560 °F). The diamond films were characterized by Scanning Electron Microscopy (SEM) and Raman spectroscopy. The spherical crystallites measured 100 to 400 nm.[43][44]
Detonation of explosives

Diamond nanocrystals (5 nm in diameter) can be formed by detonating certain carbon-containing explosives in a metal chamber. These nanocrystals are called "detonation nanodiamond". During the explosion, the pressure and temperature in the chamber become high enough to convert the carbon of the explosives into diamond. Being immersed in water, the chamber cools rapidly after the explosion, suppressing conversion of newly produced diamond into more stable graphite.[45] In a variation of this technique, a metal tube filled with graphite powder is placed in the detonation chamber. The explosion heats and compresses the graphite to an extent sufficient for its conversion into diamond.[46] The product is always rich in graphite and other non-diamond carbon forms and requires prolonged boiling in hot nitric acid (about 1 day at 250 °C) to dissolve them.[33] The recovered nanodiamond powder is used primarily in polishing applications. It is mainly produced in China, Russia and Belarus and is only now beginning to reach the market in bulk quantities.[47]
Ultrasound cavitation
Micron-sized diamond crystals can be synthesized from a suspension of graphite in organic liquid at atmospheric pressure and room temperature using ultrasonic cavitation. The diamond yield is about 10% of the initial graphite weight. The estimated cost of diamond produced by this method is comparable to that of the HPHT method, however the crystalline perfection of the produced material is significantly worse for the ultrasonic synthesis. This technique requires relatively simple equipment and procedures, but it has only been reported by two research groups, and has no industrial use as of 2009[update]. Numerous process parameters, such as preparation of the initial graphite powder, the choice of ultrasonic power, synthesis time and the solvent, are not yet optimized, leaving a window for potential improvement of the efficiency and reduction of the cost of the ultrasonic synthesis.[34][48]
Properties
Traditionally, the absence of crystal flaws is considered to be the most important quality of a diamond. Purity and high crystalline perfection make diamonds transparent and clear, whereas its hardness, optical dispersion (luster) and chemical stability (combined with marketing), make it a popular gemstone. High thermal conductivity is also important for technical applications. Whereas high optical dispersion is an intrinsic property of all diamonds, their other properties vary depending on how the diamond was created.[49]
Crystallinity
Diamond can be one single, continuous crystal or it can be made up of many smaller crystals (polycrystal). Large, clear and transparent single-crystal diamond is typically used in gemstones. Polycrystalline diamond consists of numerous small grains, which are easily seen by naked eye through strong light absorption and scattering; it is unsuitable for gems and is used for industrial applications such as mining and cutting tools. Within polycrystalline diamond, the diamond is often described by the average size (or grain size) of the crystals that make it up. Grain sizes range from nanometers to hundreds of micrometers, usually referred to as "nanocrystalline" and "microcrystalline" diamond, respectively.[50]
Hardness
Synthetic diamond is the hardest material known,[51] where hardness is defined as resistance to scratching and is graded between 1 (softest) and 10 (hardest) using the Mohs scale of mineral hardness. Diamond has a hardness of 10 (hardest) on this scale.[52] The hardness of synthetic diamond depends on its purity, crystalline perfection and orientation: hardness is higher for flawless, pure crystals oriented to the <111> direction (along the longest diagonal of the cubic diamond lattice).[53] Nanocrystalline diamond produced through CVD diamond growth can have a hardness ranging from 30% to 75% of that of single crystal diamond, and the hardness can be controlled for specific applications. Some synthetic single-crystal diamonds and HPHT nanocrystalline diamonds (see hyperdiamond) are harder than any known natural diamond.[54][55][51]
Impurities and inclusions
Every diamond contains atoms other than carbon in concentrations detectable by analytical techniques. Those atoms can aggregate into macroscopic phases called inclusions. While impurities are generally avoided, they can be introduced intentionally as a way to control certain properties of the diamond. For instance, pure diamond is an electrical insulator, but diamond with boron added is an electrical conductor (and, in some cases, a superconductor),[56] allowing it to be used in electronic applications. Nitrogen impurities hinder movement of lattice dislocations (defects within the crystal structure) and put the lattice under compressive stress, thereby increasing hardness and toughness.[57]
Thermal conductivity
Unlike most electrical insulators, pure diamond is a good conductor of heat because of the strong covalent bonding within the crystal. The thermal conductivity of pure diamond is the highest among any known solid. However, it is reduced by the small amount (1.1 at. %) of 13C isotope naturally present; 13C acts as an inhomogeneity in the 12C diamond lattice, thereby reducing its thermal conduction. Therefore, single crystals of synthetic diamond enriched in 12C isotope (99.9%) have the highest thermal conductivity of any material, 30 W/cm·K at room temperature, five times higher than copper.[58]
Diamond's thermal conductivity is made use of by jewelers and gemologists who may employ an electronic thermal probe to separate diamonds from their imitations. These probes consist of a pair of battery-powered thermistors mounted in a fine copper tip. One thermistor functions as a heating device while the other measures the temperature of the copper tip: if the stone being tested is a diamond, it will conduct the tip's thermal energy rapidly enough to produce a measurable temperature drop. This test takes about 2–3 seconds.[59]
Applications
Machining and cutting tools

Most industrial applications of synthetic diamond have long been associated with their hardness; this property makes diamond the ideal material for machine tools and cutting tools. As the hardest known naturally occurring material, diamond can be used to polish, cut, or wear away any material, including other diamonds. Common industrial adaptations of this ability include diamond-tipped drill bits and saws, and the use of diamond powder as an abrasive.[60] These are by far the largest industrial applications of synthetic diamond. While natural diamond is also used for these purposes, synthetic HPHT diamond is more popular, mostly because of better reproducibility of its mechanical properties. Diamond is however not suitable for machining ferrous alloys at high speeds, as carbon is soluble in iron at the high temperatures created by high-speed machining, leading to greatly increased wear on diamond tools when compared to alternatives.[61]
The usual form of diamond in cutting tools is micrometer-sized grains dispersed in a metal matrix (usually cobalt) sintered onto the tool. This is typically referred to in industry as polycrystalline diamond (PCD). PCD-tipped tools can be found in mining and cutting applications. For the past fifteen years, work has been done to coat metallic tools with CVD diamond, and though the work still shows promise it has not significantly replaced traditional PCD tools.[62]
Thermal conductor
Whereas most materials which have high thermal conductivity are electrically conductive (metals), pure synthetic diamond has both excellent thermal conductivity and negligible electrical conductivity. This combination is invaluable for electronics where diamond is used as a heat sink for high-power semiconductor lasers, laser arrays and high-power transistors. Efficient dissipation of heat prolongs the lifetime of those devices, and their high cost justifies the use of efficient, though relatively expensive, diamond heat sinks.[63][64] In semiconductor technology, synthetic diamond heat spreaders prevent silicon and other semiconducting materials from overheating.[65]
Optical material
Diamond is hard, chemically inert, and has high thermal conductivity and a low coefficient of thermal expansion. These properties make diamond superior to any other existing window material used for transmitting infrared and microwave radiation. Therefore, synthetic diamond is starting to replace zinc selenide as the output window of high-power CO2 lasers[66] and gyrotrons. Those synthetic diamond windows are shaped as disks of large diameters (about 10 cm for gyrotrons) and small thicknesses (to reduce absorption) and can only be produced with the CVD technique.[67][68]
Recent advances in the HPHT and CVD synthesis techniques improved the purity and crystallographic structure perfection of single-crystalline diamond enough to replace silicon as a diffraction grating and window material in high-power radiation sources, such as synchrotrons.[69][70] Both the CVD and HPHT processes are also used to create designer optically transparent diamond anvils as a tool for measuring electric and magnetic properties of materials at ultra high pressures using a diamond anvil cell.[71]
Electronics
Synthetic diamond has potential uses as a semiconductor,[72] because it can be doped with impurities like boron and phosphorus. Since these elements contain one more or one less valence electron than carbon, they turn synthetic diamond into p-type or n-type semiconductor. Making a p–n junction by sequential doping of synthetic diamond with boron and phosphorus produces light emitting diodes (LEDs) producing UV light of 235 nm.[73] Another useful property of synthetic diamond for electronics is high carrier mobility, which reaches 4500 cm2/(V·s) in single-crystal CVD diamond.[74] High mobility is favorable for high-frequency field-effect transistors. The wide band gap of diamond (5.5 eV) gives it excellent dielectric properties. Combined with the high mechanical stability of diamond, those properties are being used in prototype high-power switches for power stations.[75]
Conductive CVD diamond is a useful electrode under many circumstances.[76] Photochemical methods have been developed for covalently linking DNA to the surface of polycrystalline diamond films produced through CVD. Such DNA modified films can be used for detecting various biomolecules, which would interact with DNA thereby changing electrical conductivity of the diamond film.[77] In addition, diamonds can be used to detect redox reactions that cannot ordinarily be studied and in some cases degrade redox-reactive organic contaminants in water supplies. Because diamond is mechanically and chemically stable, it can be used as an electrode under conditions that would destroy traditional materials. As an electrode, synthetic diamond can be used in waste water treatment of organic effluents[78] and the production of strong oxidants.[79]
Synthetic diamond transistors have been produced in the laboratory and are functional at much higher temperatures than silicon devices and are resistant to chemical and radiation damage. While no diamond transistors have yet been successfully integrated into commercial electronics, they are promising for use in exceptionally high power situations and hostile environments.[80]
Synthetic diamond is already used as radiation detection device. It is radiation hard and has a wide bandgap of 5.5 eV (at room temperature). Because of these properties, it is employed in applications such as the BaBar detector at the Stanford Linear Accelerator[81] and BOLD (Blind to the Optical Light Detectors for VUV solar observations).[82][83]
Gemstones

Synthetic diamonds for use as gemstones are grown by HPHT[27] or CVD[84] methods. They are available in yellow and blue colors, and to a lesser extent colorless (or white). The yellow color comes from nitrogen impurities in the manufacturing process while the blue color comes from boron.[25] Other colors such as pink or green are achievable after synthesis using irradiation.[85] Several companies also offer memorial diamonds grown using cremated remains.[86]
Gem-quality diamonds grown in a lab can be chemically, physically and optically identical to naturally occurring ones, although they can be distinguished by spectroscopy in infrared, ultraviolet, or X-ray wavelengths. The DiamondView tester from De Beers uses UV fluorescence to detect trace impurities of nitrogen, nickel or other metals in HPHT or CVD diamonds.[87]
The mined diamond industry is evaluating marketing and distribution countermeasures to the appearance of the relatively cheap synthetic diamonds on the gem market. In an attempt to maintain high prices for diamonds, the three largest distributors of natural diamonds have made public statements about selling their diamonds with full disclosure of the individual diamond history, and have implemented measures to laser-inscribe serial numbers on their gemstones.[84]
See also
- Material properties of diamond
- Crystallographic defects in diamond
- BARS (Diamonds)
- Detonation nanodiamond
- Diamond simulant
- Moissanite
- Poly(hydridocarbyne)
- Covalent superconductors
- Isotopically pure diamond
References
- ^ J. B. Hannay (1879). "On the Artificial Formation of the Diamond". Proc. R. Soc. Lond. 30: 450–461. doi:10.1098/rspl.1879.0144.
- ^ C. Royère (1999). "The electric furnace of Henri Moissan at one hundred years: connection with the electric furnace, the solar furnace, the plasma furnace?". Annales pharmaceutiques françaises. 57: 116. PMID 10365467.
- ^ H. Moissan (1894). "Nouvelles expériences sur la reproduction du diamant". Comptes Rendus. 118: 320.
- ^ O. Ruff (1917). "Über die Bildung von Diamanten". Zeitschrift für anorganische und allgemeine Chemie. 99 (1): 73–104. doi:10.1002/zaac.19170990109.
- ^ K. Nassau (1980). Gems made by Man. Chilton Book Co. pp. 12–25. ISBN 0801967732.
- ^ J. Willard Hershey (2004). The Book of Diamonds: Their Curious Lore, Properties, Tests and Synthetic Manufacture. Kessinger Publishing. pp. 123–130. ISBN 1417977159.
- ^ J. Willard Hershey PhD (1940). Book of Diamonds. Heathside Press, New York. pp. 127–132. ISBN 0486418162.
- ^ "Permanent collection". McPherson museum. Retrieved 2009-08-08.
- ^ a b K. Lonsdale (1962). "Further Comments on Attempts by H. Moissan, J. B. Hannay and Sir Charles Parsons to Make Diamonds in the Laboratory" (PDF). Nature. 196: 104. doi:10.1038/196104a0.
- ^ M. O'Donoghue (2006). Gems. Elsevier. p. 473. ISBN 0-75-065856-8.
- ^ Barnard, pp. 6–7
- ^ C. A. Parson (1907). "Some notes on carbon at high temperatures and pressures" (PDF). Proceedings of the Royal Society of London. 79a: 532. doi:10.1098/rspa.1907.0062.
- ^ C.H. Desch (1928). "The Problem of Artificial Production of Diamonds". Nature. 121: 799. doi:10.1038/121799a0.
- ^ a b R. M. Hazen (1999). The diamond makers. Cambridge University Press. pp. 100–113. ISBN 0521654742.
- ^ O'Donoghue, p. 474
- ^ H. T. Hall (1960). "Ultra-high pressure apparatus" (PDF). Rev. Sci. Instr. 31: 125. doi:10.1063/1.1716907.
- ^ F. P. Bundy, H. T. Hall, H. M. Strong and R. H. Wentorf (1955). "Man-made diamonds". Nature. 176: 51.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b H. P. Bovenkerk, F. P. Bundy, H. T. Hall, H. M. Strong and R. H. Wentorf (1959). "Preparation of diamond". Nature. 184: 1094.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Barnard, pp. 40–43
- ^ "ACS Award for Creative Invention". American Chemical Society. Retrieved 2009-08-08.
- ^ H. Liander and E. Lundblad (1955). "Artificial diamonds". ASEA Journal. 28: 97.
- ^ Barnard, pp. 31–33
- ^ General Electric v. Sung, 843 F. Supp. 776: "granting production injunction against Iljin Diamond" cited in M. A. Epstein (1998). Epstein on intellectual property. Aspen Publishers Online. p. 121. ISBN 0735503192.
- ^ Wm. C. Hannas (2003). The writing on the wall. University of Pennsylvania Press. pp. 76–77. ISBN 0812237110.
- ^ a b R. C. Burns, V. Cvetkovic and C. N. Dodge (1990). "Growth-sector dependence of optical features in large synthetic diamonds". Journal of Crystal Growth. 104: 257. doi:10.1016/0022-0248(90)90126-6.
- ^ Barnard, p. 166
- ^ a b R. Abbaschian; et al. (2005). "High pressure-high temperature growth of diamond crystals using split sphere apparatus". Diam. Rel. Mater. 14: 1916. doi:10.1016/j.diamond.2005.09.007.
{{cite journal}}: Explicit use of et al. in:|author=(help) - ^ W. G. Eversole "Synthesis of diamond" U.S. patent 3,030,188, April 17, 1962
- ^ J. C. Angus; et al. (1968). "Growth of Diamond Seed Crystals by Vapor Deposition". J. Appl. Phys. 39: 2915. doi:10.1063/1.1656693.
{{cite journal}}: Explicit use of et al. in:|author=(help) - ^ B.V. Deryagin and D. V. Fedoseev (1970). "Epitaxial Synthesis of Diamond in the Metastable Region". Rus. Chem. Rev. 39. 39: 783. doi:10.1070/RC1970v039n09ABEH002022.
- ^ Spear and Dismukes, pp. 265–266
- ^ a b c M Werner; et al. (1998). "Growth and application of undoped and doped diamond films". Rep. Prog. Phys. 61: 1665. doi:10.1088/0034-4885/61/12/002.
{{cite journal}}: Explicit use of et al. in:|author=(help) - ^ a b Osawa, E (2007). "Recent progress and perspectives in single-digit nanodiamond". Diamond and Related Materials. 16: 2018. doi:10.1016/j.diamond.2007.08.008.
- ^ a b E. M. Galimov; et al. (2004). "Experimental Corroboration of the Synthesis of Diamond in the Cavitation Process". Doklady Physics. 49: 150. doi:10.1134/1.1710678.
{{cite journal}}: Explicit use of et al. in:|author=(help) - ^ "HPHT synthesis". International Diamond Laboratories. Retrieved 2009-05-05.
- ^ Barnard, p. 150
- ^ a b E. Ito (2007). G. Schubert (ed.). Multianvil cells and high-pressure experimental methods, in Treatise of Geophysics. Vol. 2. Elsevier, Amsterdam. p. 197–230. ISBN 0812922751.
- ^ H. T. Hall (1958). "Ultrahigh-Pressure Research: At ultrahigh pressures new and sometimes unexpected chemical and physical events occur" (PDF). Science. 128: 445. doi:10.1126/science.128.3322.445.
- ^ M. G. Loshak and L. I. Alexandrova (2001). "Rise in the efficiency of the use of cemented carbides as a matrix of diamond-containing studs of rock destruction tool". Int. J. Refractory Metals and Hard Materials. 19: 5. doi:10.1016/S0263-4368(00)00039-1.
- ^ N. Pal'yanov; et al. (2002). "Fluid-bearing alkaline carbonate melts as the medium for the formation of diamonds in the Earth's mantle: an experimental study". Lithos. 60: 145. doi:10.1016/S0024-4937(01)00079-2.
{{cite journal}}: Explicit use of et al. in:|author=(help) - ^ a b S. Koizumi, C. E. Nebel and M. Nesladek (2008). Physics and Applications of CVD Diamond. Wiley VCH. p. 50. ISBN 3527408010.
{{cite book}}: More than one of|pages=and|page=specified (help) - ^ Barjon, J. (2005). "Silicon incorporation in CVD diamond layers". Physica status solidi (a). 202: 2177. doi:10.1002/pssa.200561920.
- ^ Growth of Diamond Films from Tequila, Javier Morales, Miguel Apatiga and Victor M. Castano, 2008, arXiv:0806.1485, accessed 2009-10-20
- ^ Winners of the Ig® Nobel Prize, Ig Nobel Prize, 2009, accessed 2009-10-20
- ^ Iakoubovskii, K (2000). "Structure and defects of detonation synthesis nanodiamond". Diamond and Related Materials. 9: 861. doi:10.1016/S0925-9635(99)00354-4.
- ^ Decarli, Ps; Jamieson, Jc (1961). "Formation of Diamond by Explosive Shock". Science. 133 (3467): 1821–1822. doi:10.1126/science.133.3467.1821. PMID 17818997.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ V. Yu. Dolmatov (2006). "Development of a rational technology for synthesis of high-quality detonation nanodiamonds". Russian Journal of Applied Chemistry. 79: 1913. doi:10.1134/S1070427206120019.
- ^ A. Kh. Khachatryan; et al. (2008). "Graphite-to-diamond transformation induced by ultrasonic cavitation". Diam. Relat. Mater. 17: 931. doi:10.1016/j.diamond.2008.01.112.
{{cite journal}}: Explicit use of et al. in:|author=(help) - ^ Spear and Dismukes, pp. 308–309
- ^ Cynthia G. Zoski (2007). Handbook of electrochemistry. Elsevier. p. 136. ISBN 0444519580.
- ^ a b V. Blank; et al. (1998). "Ultrahard and superhard phases of fullerite C60: comparison with diamond on hardness and wear" (PDF). Diamond and Related Materials. 7: 427. doi:10.1016/S0925-9635(97)00232-X.
{{cite journal}}: Explicit use of et al. in:|author=(help) - ^ P. G. Read (2005). Gemmology. Butterworth-Heinemann. pp. 49–50. ISBN 0750664495.
- ^ A. J. Neves and M. H. Nazaré (2001). Properties, Growth and Applications of Diamond. IET. pp. 142–147. ISBN 0852967853.
- ^ H. Sumiya (2005). "Super-hard diamond indenter prepared from high-purity synthetic diamond crystal". Rev. Sci. Instrum. 76: 026112. doi:10.1063/1.1850654.
- ^ C-S Yan; et al. (2005). "Ultrahard diamond single crystals from chemical vapor deposition". Phys. Stat. Solidi (a). 201: R25. doi:10.1002/pssa.200409033.
{{cite journal}}: Explicit use of et al. in:|author=(help) - ^ E. Ekimov; et al. (2004). "Superconductivity in diamond" (PDF). Nature. 428: 542. doi:10.1038/nature02449.
{{cite journal}}: Explicit use of et al. in:|author=(help) - ^ S. A. Catledge (1999). "Effect of nitrogen addition on the microstructure and mechanical properties of diamond films grown using high-methane concentrations". Journal of Applied Physics. 86: 698. doi:10.1063/1.370787.
- ^ L. Wei; et al. (1993). "Thermal conductivity of isotopically modified single crystal diamond". Phys. Rev. Lett. 70: 3764. doi:10.1103/PhysRevLett.70.3764.
{{cite journal}}: Explicit use of et al. in:|author=(help) - ^ J. F. Wenckus "Method and means of rapidly distinguishing a simulated diamond from natural diamond" U.S. patent 4,488,821 December 18, 1984
- ^ C. Holtzapffel (1856). Turning And Mechanical Manipulation. Holtzapffel & Co. pp. 176–178.
- ^ R. T. Coelho; et al. (1995). "The application of polycrystalline diamond (PCD) tool materials when drilling and reaming aluminum-based alloys including MMC". International journal of machine tools & manufacture. 35: 761. doi:10.1016/0890-6955(95)93044-7.
{{cite journal}}: Explicit use of et al. in:|author=(help) - ^ W. Ahmed; et al. (2003). "Diamond films grown on cemented WC-Co dental burs using an improved CVD method". Diamond and Related Materials. 12: 1300. doi:10.1016/S0925-9635(03)00074-8.
{{cite journal}}: Explicit use of et al. in:|author=(help) - ^ "CVD Thick-Film Diamond Heat Spreaders". sp3 diamond technologies. Retrieved 2009-05-05.
- ^ M. Sakamoto, J. G. Endriz, D. R. Scifres (1992). "120 W CW output power from monolithic AlGaAs (800 nm) laser diode array mounted on diamond heatsink". Electronics Letters. 28 (2): 197–199. doi:10.1049/el:19920123.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Kramadhati V. Ravi et al. "Diamond-silicon hybrid integrated heat spreader" U.S. patent 6,924,170, August 2, 2005
- ^ D. C. Harris (1999). Materials for infrared windows and domes: properties and performance. SPIE Press. pp. 303–334. ISBN 0819434825.
- ^ T. Inai; et al. (1999). "The diamond window for a milli-wave zone high power electromagnetic wave output". New Diamond. 15: 27.
{{cite journal}}: Explicit use of et al. in:|author=(help) - ^ G. S. Nusinovich (2004). Introduction to the physics of gyrotrons. JHU Press. p. 229. ISBN 0801879213.
- ^ A. M. Khounsary; et al. "Diamond Monochromator for High Heat Flux Synchrotron X-ray Beams". Argonne National Laboratory. Retrieved 2009-05-05.
{{cite web}}: Explicit use of et al. in:|author=(help) - ^ J. Heartwig; et al. "Diamonds for Modern Synchrotron Radiation Sources". European Synchrotron Radiation Facility. Retrieved 2009-05-05.
{{cite web}}: Explicit use of et al. in:|author=(help) - ^ Jackson, D. D. (2003). "Magnetic susceptibility measurements at high pressure using designer diamond anvils". Rev. Sci. Instrum. 74: 2467. doi:10.1063/1.1544084.
{{cite journal}}: Unknown parameter|name=ignored (help) - ^ A. Denisenko and E. Kohn (2005). "Diamond power devices. Concepts and limits". Diamond and Related Materials. 14: 491. doi:10.1016/j.diamond.2004.12.043.
- ^ S. Koizumi; et al. (2001). "Ultraviolet Emission from a Diamond pn Junction". Science. 292: 1899. doi:10.1126/science.1060258.
{{cite journal}}: Explicit use of et al. in:|author=(help) - ^ J. Isberg; et al. (2002). "High Carrier Mobility in Single-Crystal Plasma-Deposited Diamond". Science. 297: 5587. doi:10.1126/science.1074374.
{{cite journal}}: Explicit use of et al. in:|author=(help) - ^ J. Isberg, M. Gabrysch, A. Tajani, and D.J. Twitchen (2006). "High-field Electrical Transport in Single Crystal CVD Diamond Diodes". Advances in Science and Technology. 48: 73. doi:10.4028/www.scientific.net/AST.48.73.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ M. Panizza and G. Cerisola (2005). "Application of diamond electrodes to electrochemical processes". Electrochimica Acta. 51: 191. doi:10.1016/j.electacta.2005.04.023.
- ^ C. E. Nebel; et al. (2007). "Inhomogeneous DNA bonding to polycrystalline CVD diamond". Diamond and Related Materials. 16: 1648. doi:10.1016/j.diamond.2007.02.015.
{{cite journal}}: Explicit use of et al. in:|author=(help) - ^ D. Gandini, E. Mahé, P.A. Michaud, W. Haenni, A. Perret, Ch. Comninellis (2000). "Oxidation of carbonylic acids at boron-doped diamond electrodes for wastewater treatment". Journal of Applied Electrochemistry. 20: 1345. doi:10.1023/A:1026526729357.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ P.A. Michaud, E. Mahé, W. Haenni, A. Perret, Ch. Comninellis (2000). "Preparation of peroxodisulfuric acid using Boron-Doped Diamond thin film electrodes". Electrochemical and Solid-State Letters. 3: 77. doi:10.1149/1.1390963.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ T. A. Railkar; et al. (2000). "A critical review of chemical vapor-deposited (CVD) diamond for electronic applications". Critical Reviews in Solid State and Materials Sciences. 25: 163. doi:10.1080/10408430008951119.
{{cite journal}}: Explicit use of et al. in:|author=(help) - ^ M. Bucciolini (2005). "Diamond dosimetry: Outcomes of the CANDIDO and CONRADINFN projects". Nuclear Instruments and Methods A. 552: 189. doi:10.1016/j.nima.2005.06.030.
- ^ "Blind to the Optical Light Detectors". Royal Observatory of Belgium. Retrieved 2009-05-05.
- ^ A. BenMoussa; et al. (2008). "New developments on diamond photodetector for VUV Solar Observations". Semiconductor Science and Technology. 23: 035026. doi:10.1088/0268-1242/23/3/035026.
{{cite journal}}: Explicit use of et al. in:|author=(help) - ^ a b A. Yarnell (February 2, 2004). "The many facets of man-made diamonds". Chemical and Engineering News. American Chemical Society. pp. 26–31. Retrieved 2009-05-04.
- ^ J. Walker (1979). "Optical absorption and luminescence in diamond". Rep. Prog. Phys. 42: 1605. doi:10.1088/0034-4885/42/10/001.
- ^ "Memorial Diamonds Deliver Eternal Life". Reuters. June 23, 2009. Retrieved 2009-08-08.
- ^ O'Donoghue, p. 115
Bibliography
- A. S. Barnard (2000). The diamond formula: diamond synthesis-a gemological perspective. Butterworth-Heinemann. ISBN 0750642440.
- Michael O'Donoghue (2006). Gems: their sources, descriptions and identification. Butterworth-Heinemann. ISBN 0750658568.
- K. E. Spear and J. P. Dismukes (1994). Synthetic diamond. Wiley-IEEE. ISBN 0471535893.
External links
- "High-pressure web server (free-download HPHT literature)".
- J. Davis (2003). "The New Diamond Age". Wired Magazine (11.09). Retrieved 2009-06-06.
- A. Yarnell (2004). "The Many Facets of Man-Made Diamonds". Chemical and Engineering News. 82: 26–31.
- "Carnegie Institution's Geophysical Laboratory". Retrieved 2009-05-05.
- "Putting the Squeeze on Materials". Retrieved 2009-05-05.
