Limbic system
This article's factual accuracy may be compromised due to out-of-date information. (November 2010) |
| Limbic system | |
|---|---|
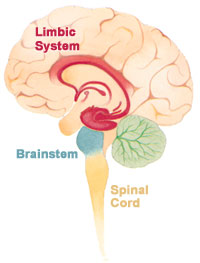 The limbic system within the brain | |
| Details | |
| Identifiers | |
| Latin | Systema limbicum |
| MeSH | D008032 |
| NeuroNames | 2055 |
| FMA | 242000 |
| Anatomical terms of neuroanatomy | |
The limbic system (or paleomammalian brain) is a complex set of brain structures that lies on both sides of the thalamus, right under the cerebrum.[1] It is not a separate system, but a collection of structures from the telencephalon, diencephalon, and mesencephalon.[2]
The limbic system includes the hippocampus, amygdala, anterior thalamic nuclei, septum, habenula, limbic cortex and fornix.
It supports a variety of functions, including emotion, behavior, motivation, long-term memory, and olfaction.[3] It appears to be primarily responsible for our emotional life, and has a great deal to do with the formation of memories.
Some neuroscientists, including Joseph LeDoux, have suggested that the concept of a functionally unified limbic system should be abandoned as obsolete because it is grounded mainly in historical concepts of brain anatomy that are no longer accepted as accurate.[4]
Etymology
The term "limbic" comes from the Latin limbus, for "border" or "edge", or, particularly in medical terminology, a border of an anatomical component. Paul Broca coined the term based on its physical location in the brain, sandwiched between two functionally different components.
Anatomy
The limbic system is the set of brain structures that forms the inner border of the cortex. The components of the limbic system located in the cerebral cortex generally have fewer layers than the classical 6-layered neocortex, and are usually classified as allocortex or archicortex.
The limbic system includes many structures in the cerebral pre-cortex and sub-cortex of the brain. The term has been used within psychiatry and neurology, although its exact role and definition have been revised considerably since the term was introduced.[5] The following structures are, or have been considered to be, part of the limbic system:
- Hippocampus and associated structures:
- Hippocampus:[6][7][8] Required for the formation of long-term memories and implicated in maintenance of cognitive maps for navigation. The hippocampus consists of two “horns” that curve back from the amygdalae. It appears to be very important in converting things that are “on one's mind” at the moment (in short-term memory) into things that one will remember for the long run (long-term memory). If the hippocampus is damaged, a person cannot build new memories and lives instead in a strange world where everything he or she experiences just fades away, even while older memories from the time before the damage are untouched (a condition depicted in the films Memento and 50 First Dates[1]).
- Amygdala:[6][7][8] Involved in signaling the cortex of motivationally significant stimuli such as those related to reward and fear in addition to social functions such as mating. Furthermore, the anatomy of amygdalae are two almond-shaped masses of neurons on either side of the thalamus at the lower end of the hippocampus. The amygdalae stimulate the hippocampus to remember many details surrounding the situation, as well.[1]
- Fornix:[6][8] is a C-shape bundle of axon that carries signals from the hippocampus to the mammillary bodies and septal nuclei.
- Mammillary body:[6] locates at the ends of the anterior arches of the fornix. It is involved with the process of recognition memory.
- Septal nuclei: Located anterior to the interventricular septum, the septal nuclei provide critical interconnections. The septal area isn't related to the sense of smell, but is the pleasure zone in animals.
- Limbic lobe
- Parahippocampal gyrus:[7] Plays a role in the formation of spatial memory.
- Cingulate gyrus:[6][7][8] Autonomic functions regulating heart rate, blood pressure and cognitive and attentional processing.
- Dentate gyrus:[7] thought to contribute to new memories.
In addition, these structures are sometimes also considered to be part of the limbic system:
- Entorhinal cortex: Important memory and associative components.
- Piriform cortex:[8] The function of which relates to the olfactory system.
- Fornicate gyrus: Region encompassing the cingulate, hippocampus, and parahippocampal gyrus.
- Nucleus accumbens: Involved in reward, pleasure, and addiction.
- Orbitofrontal cortex: Required for decision making.
Function
The hypothalamus is a part of the limbic system, which is a group of forebrain structures that has the hypothalamus, the amygdala, and the hippocampus. These are involved in motivation, emotion, learning, and memory. The limbic system is where the subcortical structures meet the cerebral cortex.[1] The limbic system operates by influencing the endocrine system and the autonomic nervous system. It is highly interconnected with the nucleus accumbens, the brain's pleasure center, which plays a role in sexual arousal and the "high" derived from certain recreational drugs. These responses are heavily modulated by dopaminergic projections from the limbic system. In 1954, Olds and Milner found that rats with metal electrodes implanted into their nucleus accumbens, as well as their septal nuclei, repeatedly pressed a lever activating this region, and did so in preference to eating and drinking, eventually dying of exhaustion.[9] The limbic system also includes the basal ganglia. The basal ganglia is a set of subcortical structures that directs intentional movements. The basal ganglia are located near the thalamus and hypothalamus. They receive input from the cerebral cortex, which sends outputs to the motor centers in the brain stem. A part of the basal ganglia called the striatum controls posture and movement. Recent studies indicate that, if there is an inadequate supply of dopamine, the striatum is affected, which can lead to visible behavioral symptoms of Parkinson’s.[1] The limbic system is also tightly connected to the prefrontal cortex. Some scientists contend that this connection is related to the pleasure obtained from solving problems. To cure severe emotional disorders, this connection was sometimes surgically severed, a procedure of psychosurgery, called a prefrontal lobotomy (this is actually a misnomer). Patients having undergone this procedure often became passive and lacked all motivation.
Cognitive components of the limbic system
Hippocampus
Spatial memory
The hippocampus has been demonstrated to be involved in various processes of cognition. The first and most widely researched area concerns memory, spatial memory in particular. Spatial memory was found to have many sub-regions in the hippocampus, such as the dentate gyrus (DG) in the dorsal hippocampus, the left hippocampus, and the parahippocampal region. The dorsal hippocampus was found to be an important component for the generation of new neurons, called adult-born granules (GC), in adolescence and adulthood.[10] These new neurons contribute to pattern separation in spatial memory, increasing the firing in cell networks, and overall causing stronger memory formations. While the dorsal hippocampus is involved in spatial memory formation, the left hippocampus is a participant in the recall of these spatial memories. Eichenbaum[11] and his team found, when studying the hippocampal lesions in rats, that the left hippocampus is “critical for effectively combining the ‘what, ‘when,’ and ‘where’ qualities of each experience to compose the retrieved memory.” This makes the left hippocampus a key component in the retrieval of spatial memory. However, Spreng[12] found that the left hippocampus is, in fact, a general concentrated region for binding together bits and pieces of memory composed not only by the hippocampus, but also by other areas of the brain to be recalled at a later time. Eichenbaum’s research in 2007 also demonstrates that the parahippocampal area of the hippocampus is another specialized region for the retrieval of memories just like the left hippocampus.
Learning
The hippocampus, over the decades, has also been found to have a huge impact in learning. CurlikShors[13] examined the effects of neurogenesis in the hippocampus and its effects on learning. This researcher and his team employed many different types of mental and physical training on their subjects, and found that the hippocampus is highly responsive to these latter tasks. Thus, they discovered an upsurge of new neurons and neural circuits in the hippocampus as a result of the training, causing an overall improvement in the learning of the task. This neurogenesis contributes to the creation of adult-born granules cells (GC), cells also described by Eichenbaum[11] in his own research on neurogenesis and its contributions to learning. The creation of these cells exhibited “enhanced excitability” in the dentate gyrus (DG) of the dorsal hippocampus, impacting the hippocampus and its contribution to the learning process.[11]
Hippocampal damage
Damage relayed to the hippocampal region of the brain has reported vast effects on overall cognitive functioning, particularly memory such as spatial memory. As previously mentioned, spatial memory is a cognitive function greatly intertwined with the hippocampus. While damage to the hippocampus may be a result of a brain injury or other injuries of that sort, researchers particularly investigated the effects that high emotional arousal and certain types of drugs had on the recall ability in this specific memory type. In particular, in a study performed by Parkard,[14] rats were given the task to correctly make their way through a maze. In the first condition, rats were stressed by shock or restraint, causing a high emotional arousal. When completing the maze task, these rats, compared to the control group, had an impaired effect on their hippocampal-dependent memory. Then, in a second condition, a group of rats were injected with anxiogenic drugs. Like the latter, these results reported similar outcomes, in that hippocampal-memory was also impaired. Studies such as these reinforce the impact that the hippocampus has on memory processing, in particular the recall function of spatial memory. Furthermore, impairment to the hippocampus can occur from prolonged exposure stress hormones, such as Glucocorticoids (GCs), which target the hippocampus and cause disruption in explicit memory.[15]
Amygdala
Episodic-autobiographical memory (EAM) networks
The amygdala, another integrative part of the limbic system, is also involved in many cognitive processes. Just as in the hippocampus, memory seems to be impacted by processes in the amygdale; however, it is not spatial memory as in the hippocampus, but episodic-autobiographical memory (EAM) networks. The amygdala, as researched by Markowitsch,[16] was found to be responsible for the encoding, storage, and retrieval of these types of memories. To delve deeper into these types of processes by the amygdala, Markowitsch[16] and his team provided extensive evidence through investigations that the “amygdala’s main function is to charge cues so that mnemonic events of a specific emotional significance can be successfully searched within the appropriate neural nets and re-activated.” These cues for emotional events created by the amygdala encompass the EAM networks previously mentioned.
Attentional and emotional processes
Besides memory, the amygdala also seems to be an important brain region involved in attentional and emotional processes. First, to define attention in cognitive terms, attention is the ability to home in on some stimuli while ignoring others. Thus, the amygdala seems to be an important structure in this ability. Foremost, however, this structure was historically thought to be linked to fear, allowing the individual to take action to rid that fear in some sort. However, as time has gone by, researchers such as Pessoa,[17] generalized this concept with help from evidence of EEG recordings, and concluded that the amygdala helps an organism to define a stimulus and therefore respond accordingly. However, when the amygdala was initially thought to be linked to fear, this gave way for research in the amygdala for emotional processes. Kheirbek[10] demonstrated research that the amygdala is involved in emotional processes, in particular the ventral hippocampus. He described the ventral hippocampus as having a role in neurogenesis and the creation of adult-born granule cells (GC). These cells not only were a crucial part of neurogenesis and the strengthening of spatial memory and learning in the hippocampus but also appear to be an essential component in the amygdala. A deficit of these cells, as Pessoa (2009) predicted in his studies, would result in low emotional functioning, leading to high retention rate of mental diseases, such as anxiety disorders.
Social processing
Social processing is an area of cognition specific to the amygdala. To be specific, the evaluation of faces in social processing is of particular importance. In a study done by Todorov,[18] fMRI tasks were performed with participants to evaluate whether the amygdala was involved in the general evaluation of faces. After the study, Todorov concluded from his fMRI results that the amygdala did indeed play a key role in the general evaluation of faces. However, in a study performed by researchers Koscik[19] and his team, the trait of truthworthiness was particularly examined in the evaluation of faces. They investigated how brain damage to the amygdala played a role in truthworthiness, and found that individuals that suffered damage tended to confuse trust and betrayal, and thus placed trust in those having done them wrong. So Koscik demonstrated that the amygdala was involved in evaluating the truthworthiness of an individual. Yet, a man named Rule,[20] along with his colleagues, expanded on the idea of the amygdala in its critique of truthworthiness in others and performed a study in 2009 in which he examined the amygdala in its role of evaluating general first impressions and relating them to real-world outcomes with his study involving first impressions of CEOs. Rule demonstrated that while the amygdala did play a role in the evaluation of truthworthiness, as observed by Koscik in his own research two years later in 2011, the amygdala played a generalized role in the overall evaluation of first impression of faces. This latter conclusion, along with Todorov’s study on the amygdala’s role in general evaluations of faces and Koscik’s research on truthworthiness and the amygdala, further solidified evidence that the amygdala plays a role in overall social processing.
The limbic system is closely related to the hypothalamus. The pituitay glad that controls the hormonal system through the hypothalamus dramatically changed through course of evolution. This allowed mammals to increase their sociality. [21]
Evolution
Paul D. MacLean, as part of his triune brain theory, hypothesized that the limbic system is older than other parts of the brain, and that it developed to manage fight or flight circuitry, which is an evolutionary necessity for reptiles as well as humans. However, recent studies of the limbic system of tetrapods have challenged some long-held tenets of forebrain evolution. The common ancestors of reptiles and mammals had a well-developed limbic system in which the basic subdivisions and connections of the amygdalar nuclei were established.[22] the human brain has acquired three components that progressively appeared and became superimposed, just as in an archeological site: the oldest, located underneath and to the back; the next one, resting on an intermediate position and the most recent, situated on top and to the front. They are, respectively:
1 - The archipallium or primitive (reptilian) brain, comprising the structures of the brain stem - medulla, pons, cerebellum, mesencephalon, the oldest basal nuclei - the globus pallidus and the olfactory bulbs. It corresponds to the reptile brain, also called "R-complex", by the famous neuroscientist Paul MacLean.
2 - The paleopallium or intermediate (old mammalian) brain, comprising the structures of the limbic system. It corresponds to the brain of the inferior mammals.
3 - The neopallium, also known as the superior or rational (new mammalian) brain, comprises almost the whole of the hemispheres (made up of a more recent type of cortex, called neocortex) and some subcortical neuronal groups. It corresponds to the brain of the superior mammals, thus including the primates and, as a consequence, the human species.
These three cerebral layers appeared, one after the other, during the development of the embryo and the fetus (ontogenesis), recapitulating, chronologically, the evolution of animal species (phylogenesis), from the lizards up to the homo sapiens. According to Maclean, they are three biological computers that, although interconnected, retained, each one, "their peculiar types of intelligence, subjectivity, sense of time and space, memory, mobility and other less specific functions".
Lesions
Damage to the structures of limbic system results in conditions like Alzheimer's disease, anterograde amnesia, retrograde amnesia, and Kluver-Bucy syndrome.
History
The French physician Paul Broca first called this part of the brain [le grand lobe limbique] Error: {{Lang}}: text has italic markup (help) in 1878.[23] He examined the differentiation between deeply recessed cortical tissue and underlying, subcortical nuclei.[24] However, most of its putative role in emotion was developed only in 1937 when the American physician James Papez described his anatomical model of emotion, the Papez circuit.[25] Paul D. MacLean expanded these ideas to include additional structures in a more dispersed "limbic system," more on the lines of the system described above.[26] The term was formally introduced by Paul D. MacLean in 1952. Paul D. MacLean developed the intriguing theory of the “triune brain” to explain its evolution and to try to reconcile rational human behavior with its more primal and violent side. He became interested in the brain’s control of emotion and behavior. After initial studies of brain activity in epileptic patients, he turned to cats, monkeys, and other models, using electrodes to stimulate different parts of the brain in conscious animals.[27] Furthermore, He then recorded the animals’ responses and, in the 1950s, he began to trace individual behaviors like aggression and sexual arousal to their physiological sources. He analyzed the brain’s center of emotions, the limbic system, and described an area that includes structures called the hippocampus and amygdala. Developing observations made by Dr. James W. Papez, he determined that the limbic system had evolved in early mammals to control fight-or-flight responses and react to both emotionally pleasurable and painful sensations. The concept is now broadly accepted in neuroscience.[28] Additionally, Dr. MacLean said that the idea of the limbic system leads to a recognition that its presence “represents the history of the evolution of mammals and their distinctive family way of life.”In the 1960s, Dr. MacLean enlarged his theory to address the human brain’s overall structure and divided its evolution into three parts, an idea that he termed the triune brain. In addition to identifying the limbic system, he pointed to a more primitive brain called the R-complex, related to reptiles, which controls basic functions like muscle movement and breathing. The third part, the neocortex, controls speech and reasoning and is the most recent evolutionary arrival.[29] The concept of the limbic system has since been further expanded and developed by Walle Nauta, Lennart Heimer and others.
Academic dispute
Still, there remains much controversy over the use of the term limbic system. When it was first coined, it was posited as the emotional center of the brain, with cognition being the business of the neocortex by contrast. However, this almost immediately ran into trouble when damage to the hippocampus, a primary limbic structure, was shown to result in severe cognitive (memory) deficits. And, since its inception, the delineating boundaries of the limbic system have been changed again and again by the neuroscience community. More recently, attempts have been made to salvage the concept through more precise definition, but there are still no generally accepted criteria for defining its parts. As a concept grounded more in tradition than in facts, some scientists have suggested that the concept should be considered obsolete and abandoned.[4]
See also
- Limbic-hypothalamic-pituitary-adrenal axis (LHPA axis)
- Emotional memory
- Fundamentals of Neuroscience at Wikiversity
- Paralimbic cortex
- Triune brain
References
- ^ a b c d e Schacter, Daniel L. 2012. Psychology.sec. 3.20
- ^ Princeton Review (29 July 2003). Anatomy Coloring Workbook, Second Edition. The Princeton Review. pp. 120–. ISBN 978-0-375-76342-7. Retrieved 10 January 2013.
- ^ Medline Plus Medical Encyclopedia
- ^ a b Ledoux, J., (2003). Synaptic Self. New York: Penguin Books. 0142001783
- ^ Conn, Michael P. 2003. Neuroscience in Medicine, 370
- ^ a b c d e Normandy
- ^ a b c d e stanford.edu
- ^ a b c d e Biology.about.com
- ^ Olds, J., Milner, P. 1954. Positive reinforcement produced by electrical stimulation of septal area and other regions of rat brain. J.Comp. Physiolo. Psycholo. 47, 419–427
- ^ a b Kheirbeck, M.A. (2011). "Dorsal vs ventral hippocampal neurogenensis: Implications for cognition and mood". Neuropsychopharmacology. 36 (1): 373–374. doi:10.1038/npp.2010.148. PMC 3055508. PMID 21116266.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ a b c Eichenbaum, H. (2007). "Comparative cognition, hippocampal function, and recollection". Comparative Cognition & Behavior Reviews. 2 (1): 47–66.
- ^ Spreng, R.N. (2012). "I remember you: A role for memory in social cognition and the functional neuroanatomy of their interaction". Brain Research. 1428: 43–50. doi:10.1016/j.brainres.2010.12.024. PMC 3085056. PMID 21172325.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ CurlikShors, D.; Shors, T.J. (2012). "Training your brain: Do mental and physical (map) training enhance cognition through the process of neurogenesis in the hippocampus?". Neuropharmacology. 64 (1): 506–14. doi:10.1016/j.neuropharm.2012.07.027. PMC 3445739. PMID 22898496.
- ^ Parkard, M.G. (2009). "Anxiety, cognition, and habit: A multiple memory systems perspective". Brain Research. 1293: 121–128. doi:10.1016/j.brainres.2009.03.029. PMID 19328775.
- ^ http://dept.wofford.edu/neuroscience/neuroseminar/pdfSpring2008/Sapolsky-2003.pdf
- ^ a b Markowitsch, H.J. (2011). "Amygdala in action: Relaying biological and social significance to autobiographical memory". Neuropsychologia. 49 (4): 718–733. doi:10.1016/j.neuropsychologia.2010.10.007. PMID 20933525.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help); horizontal tab character in|title=at position 52 (help) - ^ Pessoa, L. (2010). "Emotion and cognition and the amygdale: From "what is it?" to "what's to be done?"". Neuropsychologia. 48 (12): 3416–3429. doi:10.1016/j.neuropsychologia.2010.06.038. PMC 2949460. PMID 20619280.
{{cite journal}}: horizontal tab character in|title=at position 77 (help) - ^ Todorov, A. (2008). "). The role of the amygdala in implicit evaluation of emotionally neutral faces". Social Cognitive and Affective Neuroscience. 3 (4): 303–312. doi:10.1093/scan/nsn033. PMC 2607057. PMID 19015082.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Koscik, T.R. (2011). "The human amygdala is necessary for developing and expressing normal interpersonal trust". Neuropsychologia. 49 (4): 602–611. doi:10.1016/j.neuropsychologia.2010.09.023. PMC 3056169. PMID 20920512.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help); horizontal tab character in|title=at position 52 (help) - ^ Rule, N.O. (2011). "Face value: Amygdala response reflects the validity of first impressions". NeuroImage. 54 (1): 734–741. doi:10.1016/j.neuroimage.2010.07.007. PMID 20633663.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Jenkins, Dacher Keltner, Keith Oatley, Jennifer M. Understanding emotions (3rd ed. ed.). Hoboken, N.J.: Wiley. ISBN 9781118147436.
{{cite book}}:|edition=has extra text (help)CS1 maint: multiple names: authors list (link) - ^ Bruce LL, Neary TJ (1995). "The limbic system of tetrapods: a comparative analysis of cortical and amygdalar populations". Brain Behav. Evol. 46 (4–5): 224–34. doi:10.1159/000113276. PMID 8564465.
- ^ Broca, P. Anatomie comparée des circonvolutions cérébrales: le grand lobe limbique. Rev. Anthropol. 1878;1:385–498.
- ^ Binder, Marc D (2009). Encyclopedia of Neuroscience. Springer. p. 2592.
- ^ Papez JW. A proposed mechanism of emotion. 1937. J Neuropsychiatry Clin Neurosci. 1995;7(1):103-12. PMID 7711480
- ^ P. D. MacLean (1952). "Some psychiatric implications of physiological studies on frontotemporal portion of limbic system (visceral brain)". Electroencephalography and Clinical Neurophysiology. 4 (4): 407–418. doi:10.1016/0013-4694(52)90073-4. PMID 12998590.
- ^ Robert L, Isaacson (31 December 1992). "A fuzzy limbic system". Behavioural Brain Research. 52 (2): 129–131. doi:10.1016/S0166-4328(05)80222-0. PMID 1294191.
- ^ Simpson, J. A. (November 1973). "The Limbic System" (PDF). J Neurol Neurosurg Psychiatry. 39 (11): 1138–1138. doi:10.1136/jnnp.39.11.1138-a. Retrieved 1 December 2012.
{{cite journal}}: CS1 maint: date and year (link) - ^ Fulton, John (November 1953). "The Limbic System". Yale Journal of Biology and Medicine. 26 (2): 107–118. PMC 2599366. PMID 13123136. Retrieved 1 December 2012.
{{cite journal}}: CS1 maint: date and year (link)
