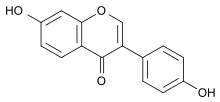
Daidzein
| |

| |
| Names | |
|---|---|
| IUPAC name
7-Hydroxy-3-(4-hydroxyphenyl) chromen-4-one
| |
| Other names
4',7-Dihydroxyisoflavone
Daidzeol Isoaurostatin | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.006.942 |
| KEGG | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C15H10O4 | |
| Molar mass | 254.23 g/mol |
| Appearance | Pale yellow prisms |
| Melting point | 315–323 °C |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Daidzein structurally belongs to the group of isoflavones.
Natural occurrences
Daidzein and other isoflavone compounds, such as genistein, are present in a number of plants and herbs like Kwao Krua (Pueraria mirifica) and Kudzu (Pueraria lobata). It can also be found in Maackia amurensis cell cultures.[2]
In food sources
Daidzein can be found in food such as soybeans and soy products like tofu and textured vegetable protein. Soy isoflavones are a group of compounds found in and isolated from the soybean. Of note, total isoflavones in soybeans are—in general—37 percent daidzein, 57 percent genistein and 6 percent glycitein, according to USDA data.[3] Soy germ contains 41.7 percent daidzein.[4]
Biological activities
Daidzein can be converted to its end metabolite S-equol in some humans based on the presence of certain intestinal bacteria. Based on several decades of research, S-equol has potential for significant health benefits.
Daidzein has no classification in the United States, where it is not considered to be generally recognized as safe (GRAS),[5] and has not been approved as a drug for any indication. It is a component of foods and dietary supplements derived from soy.[6] Dietary supplements are not regulated as drugs in the U.S., and the labeling of dietary supplements in the U.S. may not describe the supplement as having any drug activity or effectiveness.[7]
Scientists have studied some of the activities of daidzein in their laboratories, working with cells or with animals such as mice. Studies in cells and in animals sometimes give hints as to what a chemical might do when given to humans, but no one can know what a chemical does in humans until the chemical is tested in a clinical trial.
Activation of PPARs
Daidzein transactivates all three PPAR isoforms, α, δ, and γ and influences target cells.[8]
Cell proliferation studies
Daidzein has both estrogenic and anti-estrogenic effects. Experiments in cells and in animals showed that even low concentration stimulates breast tumor growth in in vitro and in vivo, and interfere with the antitumor effect of the cancer drug tamoxifen.[9] T47D:A18/PKC alpha tumor growth was demonstrated to be stimulated by genistein, but partially inhibited by daidzein; however, coadministration of tamoxifen with either daidzein or genistein produced tumors of greater size.[10]
Antioxidant
Scientific studies of daidzein's antioxidant properties have given contradictory results: some studies have shown antioxidant properties in laboratory experiments on cells, but in other experiments daidzein has caused oxidative stress on cells.[11]
Daidzein metabolite S-equol activities
Daidzein, when consumed from soy, is transformed in some humans, but not all, to produce S-equol [7-hydroxy-3-(49-hydroxyphenyl)-chroman],[12] Because it is a metabolite of daidzein, S-equol is not of plant origin. The molecular and physical structure of S-equol is similar to that of estradiol,[13] the main sex hormone found in women.
The ability to transform daidzein into S-equol is based on the presence of certain intestinal bacteria. In fact, several studies indicate that only 25 to 30 percent of the adult population of Western countries produces S-equol after eating soy foods containing isoflavones,[13][14][15][16] significantly lower than the reported 50 to 60 percent frequency of equol-producers in adults from Japan, Korea, or China.[17][18][19][20]
Although still under investigation, the ability to produce S-equol may be associated with other health benefits, according to data from epidemiological and clinical trials. Studies in both animal models and humans have yielded data about the potential of S-equol use in menopause[21][22] breast and prostate cancer,[13] and bone health.[23][24]
Glycosides
List of plants that contain the chemical
Notes and references
- ^ Merck Index, 11th Edition, 2805.
- ^ Isoflavonoid production by callus cultures of Maackia amurensis. S.A Fedoreyev, T.V Pokushalov, M.V Veselova, L.I Glebko, N.I Kulesh, T.I Muzarok, L.D Seletskaya, V.P Bulgakov and Yu.N Zhuravlev, Fitoterapia, 1 August 2000, Volume 71, Issue 4, Pages 365–372, doi:10.1016/S0367-326X(00)00129-5
- ^ "Isoflavones contents of food". Top Cultures. Retrieved 15 May 2012.
- ^ Zhang, Y. (1999). "Urinary disposition of the soybean isoflavones daidzein, genistein and glycitein differs among humans with moderate fecal isoflavone degradation activity". The Journal of Nutrition. 129 (5): 957–962. PMID 10222386.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ FDA GRAS database
- ^ Fact Sheet On The Phytoestrogen Daidzein
- ^ FDA 101: Dietary Supplements
- ^ Dang Z. C.; Löwik, C. (2004). "The Balance between Concurrent Activation of ERs and PPARs Determines Daidzein-Induced Osteogenesis and Adipogenesis". Journal of Bone and Mineral Research. 19 (5): 853–861. doi:10.1359/jbmr.040120. PMID 15068509.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ de Lemos, M. L. (2001). "Effects of soy phytoestrogens genistein and daidzein on breast cancer growth". Annals of Pharmacotherapy. 35 (9): 11118–11121. doi:10.1345/aph.10257. PMID 11573864.
- ^ Tonetti, D. A.; Zhang, Y.; Zhao, H.; Lim, S. B.; Constantinou, A. I. (2007). "The effect of the phytoestrogens genistein, daidzein, and equol on the growth of tamoxifen-resistant T47D / PKCα". Nutrition and Cancer. 58 (2): 1222–1229. doi:10.1080/01635580701328545. PMID 17640169.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Röhrdanz, E.; Ohler, S.; Tran-Thi, Q. H.; Kahl, R. (2002). "The Phytoestrogen Daidzein Affects the Antioxidant Enzyme System of Rat Hepatoma H4IIE Cells" (pdf). Journal of Nutrition. 132 (2): 370–375. PMID 11880557.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Setchell, K. D. (2010). "Equol: History, Chemistry, and Formation" (pdf). The Journal of Nutrition. 140 (7): 1355S–1362S. doi:10.3945/jn.109.119776. PMC 2884333. PMID 20519412.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ a b c Atkinson, C. (2005). "Gut bacterial metabolism of the soy isoflavone daidzein: exploring the relevance to human health" (pdf). Experimental Biology and Medicine. 230 (3): 155–170. PMID 15734719.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Lampe, J. W. (1998). "Urinary Equol Excretion with a Soy Challenge: Influence of Habitual Diet". Proceedings of the Society for Experimental Biology and Medicine. 217 (3): 335–339. PMID 9492344.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Setchell, K. D. (2006). "Method of Defining Equol-Producer Status and its Frequency among Vegetarians" (pdf). The Journal of Nutrition. 136 (8): 2188–2193. PMID 16857839.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Rowland, I. R. (2000). "Interindividual Variation in Metabolism of Soy Isoflavones and Lignans: Influence of Habitual Diet on Equol Production by the Gut Microflora". Nutrition and Cancer. 36 (1): 27–32. doi:10.1207/S15327914NC3601_5. PMID 10798213.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Watanabe, S. (1998). "Pharmacokinetics of Soybean Isoflavones in Plasma, Urine and Feces of Men after Ingestion of 60 g Baked Soybean Powder (Kinako)" (pdf). The Journal of Nutrition. 128 (10): 1710–1715. PMID 9772140.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Arai, Y. (2000). "Comparison of Isoflavones among Dietary Intake, Plasma Concentration and Urinary Excretion for Accurate Estimation of Phytoestrogen Intake" (pdf). Journal of Epidemiology. 10 (2): 127–135. PMID 10778038.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 15067102 , please use {{cite journal}} with
|pmid= 15067102instead. - ^ Song, K. B. (2006). "Prevalence of Daidzein-Metabolizing Phenotypes Differs between Caucasian and Korean American Women and Girls" (pdf). The Journal of Nutrition. 136 (5): 1347–1351. PMID 16614428.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Aso, T. (2012). "A natural S-equol supplement alleviates hot flushes and other menopausal symptoms in equol nonproducing postmenopausal Japanese women". Journal of Women's Health. 21 (1): 92–100. doi:10.1089/jwh.2011.2753. PMID 21992596.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Jou, H. J. (2008). "Effect of intestinal production of equol on menopausal symptoms in women treated with soy isoflavones". International Journal of Gynaecology and Obstetrics. 102 (1): 44–49. doi:10.1016/j.ijgo.2008.01.028. PMID 18395723.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Wu, J. (2007). "Possible role of equol status in the effects of isoflavone on bone and fat mass in postmenopausal Japanese women: a double-blind, randomized, controlled trial". Menopause. 14 (5): 866–874. doi:10.1097/gme.0b013e3180305299. PMID 17464237.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Tousen, Y. (2011). "Natural S-Equol Decreases Bone Resorption in Postmenopausal, Non-Equol-Producing Japanese Women: A Pilot Randomized, Placebo-Controlled Trial". Menopause. 18 (5): 563–574. doi:10.1097/gme.0b013e3181f85aa7. PMID 21252728.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Chen, G.; Zhang, J.; Ye, J. (2001). "Determination of Puerarin, Daidzein and Rutin in Pueraria lobata (Willd.) Ohwi by Capillary Electrophoresis with Electrochemical Detection". Journal of Chromatography A. 923 (1–2): 255–262. doi:10.1016/S0021-9673(01)00996-7. PMID 11510548.
- ^ Xu, H.-N.; He, C.-H. (2007). "Extraction of Isoflavones from Stem of Pueraria lobata (Willd.) Ohwi Using n-Butanol / Water Two-Phase Solvent System and Separation of Daidzein". Separation and Purification Technology. 56 (1): 255–262. doi:10.1016/j.seppur.2007.01.027.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Zhou, H. Y.; Wang, J. H.; Yan, F. Y. (2007). "[Separation and Determination of Puerarin, Daidzin and Daidzein in Stems and Leaves of Pueraria thomsonii by RP-HPLC]". Zhongguo Zhong Yao Za Zhi (in Chinese). 32 (10): 937–939. PMID 17655152.
{{cite journal}}: CS1 maint: multiple names: authors list (link)
