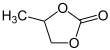
Propylene carbonate
| |||

| |||
| Names | |||
|---|---|---|---|
| IUPAC name
4-Methyl-1,3-dioxolan-2-one
| |||
| Other names
(R,S)-4-Methyl-1,3-dioxolan-2-one
Cyclic propylene carbonate Carbonic acid propylene ester Cyclic 1,2-propylene carbonate Propylene glycol cyclic carbonate 1,2-Propanediol carbonate 4-Methyl-2-oxo-1,3-dioxolane Arconate 5000 Texacar PC | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChemSpider | |||
| ECHA InfoCard | 100.003.248 | ||
PubChem CID
|
|||
| UNII | |||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| C4H6O3 | |||
| Molar mass | 102.089 g·mol−1 | ||
| Appearance | Colorless liquid | ||
| Density | 1.205 g/cm3 | ||
| Melting point | −48.8 °C (−55.8 °F; 224.3 K) | ||
| Boiling point | 242 °C (468 °F; 515 K) | ||
| Very soluble | |||
Refractive index (nD)
|
1.4189 | ||
| Hazards | |||
| Occupational safety and health (OHS/OSH): | |||
Main hazards
|
Xi | ||
| NFPA 704 (fire diamond) | |||
| Flash point | 132 °C (270 °F; 405 K) | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Propylene carbonate (often abbreviated PC) is an organic compound with the formula C4H6O3. It is a carbonate ester derived from propylene glycol.[3] This colorless and odorless liquid is useful as a polar, aprotic solvent. Propylene carbonate is chiral but is used exclusively as the racemic mixture.[4]
Preparation
Propylene carbonate is a produced from propylene oxide and carbon dioxide with a suitable catalyst, often a zinc halide. [citation needed]
- C3H6O + CO2 → C4H6O3
The propylene carbonate product may be converted to other desirable carbonate esters by transesterification as well (see Carbonate ester#Carbonate transesterification).[5]
Applications
Propylene carbonate is used in a variety of syntheses and applications as a polar, aprotic solvent. It has a high molecular dipole moment (4.9 D), considerably higher that those of acetone (2.91 D) and ethyl acetate (1.78 D).[1] It is possible, for example, to obtain potassium, sodium, and other alkali metals by electrolysis of their chlorides and other salts dissolved in propylene carbonate.[6]
Due to its high dielectric constant of 64, it is frequently used as a high-permittivity component of electrolytes in lithium batteries, usually together with a low-viscosity solvent (e.g. dimethoxyethane). Its high polarity allows it to create an effective solvation shell around lithium ions, thereby creating a conductive electrolyte. However, it is not used in lithium-ion batteries due to its destructive effect on graphite.[7]
Propylene carbonate can also be found in some adhesives, paint strippers, and in cosmetics.[8] It is also used as plasticizer.
In electrospray ionization mass spectrometry, propylene carbonate is doped into low surface tension solutions to increase analyte charging.[9]
Safety
Clinical studies indicate that propylene carbonate does not cause skin irritation or sensitization when used in cosmetic preparations, whereas moderate skin irritation is observed when used undiluted. No significant toxic effects were observed in rats fed propylene carbonate, exposed to the vapor, or exposed to the undiluted liquid.[10]
See also
References
- ^ a b Haynes, William M., ed. (2011). CRC Handbook of Chemistry and Physics (92nd ed.). Boca Raton, FL: CRC Press. ISBN 1-4398-5511-0.
- ^ Propylene carbonate at Sigma-Aldrich
- ^ WebBook page for propylene carbonate
- ^ Dieter Stoye. "Solvents". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a24_437. ISBN 978-3527306732.
- ^ Hans-Josef Buysch. "Carbonic Esters". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a05_197. ISBN 978-3527306732.
- ^ J. Jorné; C. W. Tobias (1975). "Electrodeposition of the alkali metals from propylene carbonate". J. Appl. Electrochem. 5 (4): 279–290. doi:10.1007/BF00608791.
- ^ Doron Aurbach (1999). Nonaqueous Electrochemistry. CRC Press. ISBN 978-0824773342.
- ^ Template:HPD
- ^ Teo CA, Donald WA (May 2014). "Solution additives for supercharging proteins beyond the theoretical maximum proton-transfer limit in electrospray ionization mass spectrometry". Anal. Chem. 86 (9): 4455–62. doi:10.1021/ac500304r. PMID 24712886.
- ^ "Environmental Profile for Propylene Carbonate". U.S. Environmental Protection Agency. 1998.