User:Elcap/Special capacitors
This article may require copy editing for grammar, style, cohesion, tone, or spelling. |
Niobium capacitor
[edit]A niobium electrolytic capacitor, a passive electronic component and a member of the family of electrolytic capacitors, is a polarized capacitor whose anode electrode (+) is made of passivated niobium metal or niobium monoxide on which an very thin insulating niobium pentoxide layer originates by anodically oxidation (forming), which acts as the dielectric of the niobium capacitor. A solid electrolyte which covers the surface of the oxide layer serves as the second electrode (cathode) (-) of the capacitor.
Niobium capacitors are available as SMD chip capacitors and compete with tantalum chip capacitors in certain voltage and capacitance ratings. They are available with a solid manganese dioxide as well as a solid conductive polymer electrolyte. Niobium capacitors are polarized components by the manufacturing principle and may only be operated with DC voltage in correct polarity. . Reverse voltage or ripple current higher than specified can destroy the dielectric and thus the capacitor. The destruction of dielectric may have catastrophic consequences. For safe operation of niobium capacitors special circuit design rules are specified from the manufacturers.
Niobium capacitors were developed in the Soviet Union in the 1960s. Since 2002 they have been commercially available in the West to take advantage of the lower cost and better availability of niobium compared with tantalum.
Basic information
[edit]Niobium is a sister metal to tantalum. Niobium has a similar high melting point (2744 °C) as tantalum and exhibit similar chemical properties. The materials and processes used to produce niobium-dielectric capacitors are essentially the same as for existing tantalum-dielectric capacitors. However, niobium as a raw material is much more abundant in nature than tantalum and is less expensive. The characteristics of this niobium electrolytic capacitors and tantalum electrolytic capacitors are roughly comparable.
Niobium electrolytic capacitors can be made with high purity niobium as the anode, but the diffusion of oxygen from the dielectric (Nb2O5) into the niobium anode metal is very high, resulting in leakage current instability or even capacitor failures.
There are two possible ways to reduce oxygen diffusion and improve leakage current stability – either by doping metallic niobium powders with nitride into passivated niobium nitride or using niobium oxide (NbO) as anode material. Niobium oxide is a hard ceramic material characterized by high metallic conductivity. Niobium oxide powder can be prepared in a similar structure to that of tantalum powder and can be processed in a similar way to produce capacitors. It also can be oxidized by anodic oxidation (anodizing, forming) to generate the insulating dielectric layer. So two types of niobium electrolytic capacitors are on the market, one use a passivated niobium anode the other use a niobium oxide anode both types use niobium pentoxide (Nb2O5) as dielectric layer.
Basic principle of anodic oxidation
[edit]
All electrolytic capacitors are using a chemical feature of some special metals, earlier called “valve metals”, which can form by anodically oxidation an insulating oxide layer that serves as dielectric. Applying a positive voltage to the anode material in an electrolytic bath forms an oxide barrier layer with a thickness corresponding to the applied voltage. This oxide layer acts as dielectric in an electrolytic capacitor.
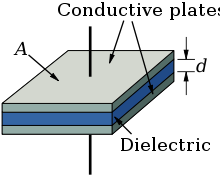
Every electrolytic capacitor in principle forms a "plate capacitor" whose capacitance is greater, the larger the electrode area (A) and the permittivity (ε) are and the thinner the thickness (d) of the dielectric is.
The dielectric thickness of niobium electrolytic capacitors is very thin in the range of nano-meter per volt. [1] With this very thin dielectric oxide layer, combined with a sufficiently high dielectric strength, the niobium electrolytic capacitors can achieve a high volumetric capacitance which is comparable to tantalum capacitors. This is one reason for the high capacitance values of electrolytic capacitors compared with other conventional capacitors.
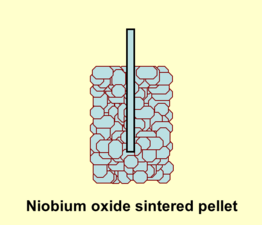
The niobium anode material is manufactured from a powder sintered into a pellet with a rough surface structure intended to increase the electrode surface A compared to a smooth surface of the same area or the same volume. That increases the later capacitance value, depending on the rated voltage, by the factor of up to 200 for solid niobium electrolytic capacitors[2]. The large surface compared to a smooth one is the second reason for the relatively high capacitance values of niobium electrolytic capacitors.
One special advantage is given for all electrolytic capacitors. Because the forming voltage defines the oxide layer thickness the voltage proof of the later electrolytic capacitor can be produced very simple for the desired rated value. That makes electrolytic capacitors fit for uses down to 2 V applications in which other capacitor technologies must stay to much higher limits.
The properties of this niobium pentoxide dielectric layer compared with tantalum pentoxide layer are given in the following table[3]:
| Anode- material |
Dielectric | Permittivity ε |
Oxide structure |
Breakdown voltage (V/µm) |
Voltage proof (nm/V) |
|---|---|---|---|---|---|
| Tantalum | Tantalum pentoxide Ta2O5 | 27 | amorphous | 625 | 1.6 |
| Niobium or Niobium oxide |
Niobium pentoxide Nb2O5 | 41 | amorphous | 400 | 2.5 |
The higher permittivity but lower breakdown voltage of niobium pentoxide in niobium capacitors results in a capacitors of similar size to those using tantalum pentoxide in tantalum capacitors.
Basic construction of solid niobium electrolytic capacitors
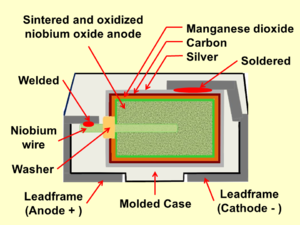
[edit]- Construction of a solid niobium chip capacitor
-
The capacitor cell of a niobium electrolytic capacitor consist out of sintered niobium or niobium monoxide powder
-
Schematic representation of the structure of a sintered niobium electrolytic capacitor with solid electrolyte and the cathode contacting layers
-
Construction of a typical SMD niobium electrolytic chip capacitor with solid electrolyte
A typical niobium capacitor is a chip capacitor and consists of niobium or niobium oxide powder pressed and sintered into a pellet as the anode of the capacitor, with the oxide layer of tantalum pentoxide as dielectric, and a solid manganese dioxide electrolyte as the cathode.
Comparison of niobium and tantalum electrolytic capacitor types
[edit]The combination of anode materials for niobium and tantalum electrolytic capacitors and the electrolytes used has formed a wide variety of capacitor types with different properties. An outline of the main characteristics of the different types is shown in the table below.
| Electrolytic capacitor family |
Electrolyte | Capacitance- range (µF) |
Max. rated voltage (V) |
Max. temperature (°C) |
|---|---|---|---|---|
| Tantalum electrolytic capacitor, sintered anode |
Non-solid, sulfuric acid | 0.1…18.000 | 630 | 125/200 |
| Solid, manganese dioxide | 0.1…3,300 | 125 | 125/150 | |
| Solid, polymer | 10…1,500 | 25 | 105 | |
| Niobium oxide- electrolytic capacitor sintered anode |
Solid,manganese dioxide | 1…1,500 | 10 | 105 |
| Solid, polymer | 4.7…470 | 16 | 105 |
Tantalum electrolytic capacitors with solid electrolyte as surface-mountable chip capacitors are mainly used in electronic devices in which little space is available or a low profile is required. They operate reliable over a wide temperature range without large parameter deviations. Niobium electrolytic capacitors are in direct competition with industrial tantalum electrolytic capacitors because niobium is more readily available. Their properties are comparable to each other.[4][3][1][5][6]
Comparison of electrical parameters of niobium and tantalum capacitor types
[edit]In order to compare the different characteristics of the different electrolytic chip capacitor types specimen with the same dimensions and of comparable capacitance and voltage are compared in the following table. In such a comparison the values for ESR and ripple current load are the most important parameters for the use of electrolytic capacitors in modern electronic equipment. The lower the ESR the higher the ripple current per volume the better the functionality of the capacitor in the circuit.
| Electrolytic capacitor family |
Type 1) | Dimension DxL, WxHxL (mm) |
Max. ESR 100 kHz, 20 °C (mΩ) |
Max. Ripple current 85/105 °C (mA) |
Max. Leakage current after 2 Min. 2) (µA) |
|---|---|---|---|---|---|
| Tantalum capacitors, MnO2 electrolyte |
Kemet T494 330/10 |
7,3x4.3x4.0 | 100 | 1285 | 10 (0.01CV) |
| Tantalum capacitors, Multianode,MnO2 electrolyte |
Kemet T510 330/10 |
7.3x4.3x4.0 | 35 | 2500 | 10 (0.01CV) |
| Tantalum capacitors, Polymer electrolyte |
Kemet T543 330/10 |
7.3x4.3x4,0 | 10 | 4900 | 100 (0.1CV) |
| Tantalum capacitors, Multianode, polymer |
Kemet T530 150/10 |
7.3x4.3x4.0 | 5 | 4970 | 100 (0.1CV) |
| Niobium capacitors, MnO2 electrolyte |
AVX,NOS 220/6,3 |
7.3x4.3x4.1 | 80 | 1461 | 20 (0.02CV) |
| Niobium capacitors, Multianode, MnO2 electrolyte |
AVX,NBM 220/6.3 |
7.3x4.3x4.1 | 40 | 2561 | 20 (0.02CV) |
| Niobium-caps Polymer electrolyte |
NEC, NMC 100/10 |
7.3x4.3x2.8 | - | - | 20 (0.02CV) |
| Aluminum capacitors, Polymer electrolyte |
Panasonic SP-UE 180/6.3 |
7.3x4.3x4.2 | 7 | 3700 | 100 (0.1CV) |
| Aluminum capacitors, Polymer electrolyte |
Kemet A700 100/10 |
7.3x4.3x4.0 | 10 | 4700 | 40 (0.04CV) |
1) 100 µF/10 V, unless otherwise specified,
2) calculated for a capacitor 100 µF/10 V,
History
[edit]See also: Electrolytic capacitor#History
The phenomenon that can form in an electrochemical process an oxide layer on aluminum and other metals like tantalum or niobium, which blocks an electric current in one direction but allows it to flow in the other direction, it was discovered in 1875 by the French researcher Eugène Ducretet. He coined the term „valve metal“ for such metals. Charles Pollak (born Karol Pollak) used this phenomenon for an idea of an polarized “Electric liquid capacitor with aluminum electrodes”. In 1896 he handed out this as patent for the first electrolytic capacitor[7]. The first tantalum electrolytic capacitors with wound tantalum foils and non-solid electrolyte were developed in 1930 by Tansitor Electronic Inc., USA, used for military purposes[8].
The relevant development of solid electrolyte tantalum capacitors began in the early 1950s as a miniaturized, more reliable low-voltage support capacitor to complement the newly invented transistor. The solution found by R. L. Taylor and H. E. Haring of the Bell Labs was based on experience with ceramics. They ground down tantalum to a powder, pressed this powder into a cylindrical form and then sintered the powder particles at high temperature between 1500 and 2000 °C under vacuum conditions to a pellet (“slug”).[9][10] These first sintered tantalum capacitors used a non-solid electrolyte, what don’t fit the concept of solid electronics. 1952 a targeted search in the Bell Labs for a solid electrolyte by D. A. McLean and F. S. Power led to the invention of manganese dioxide as a solid electrolyte for a sintered tantalum capacitor[11].
Niobium is also a so-called valve metal such as tantalum and aluminum, on which an electrically insulating oxide layer is formed by anodic oxidation, if a positive voltage is applied. For niobium this behavior was known since the beginning of the 20th century. The high melting point of 2744 °C hindered the development of niobium electrolytic capacitors. Niobium is more abundant in nature than tantalum and is less expensive. In the 1960s, the better availability of niobium ore compared with tantalum ore prompted research into niobium electrolytic capacitors in the former Soviet Union.[12] Here they took the place that was filled by tantalum capacitors in the West.
With the collapse of the Iron Curtain, this know-how has been publicized in the West. In the late 1990s the interest in this technology awoke in the major capacitor manufacturers. The materials and processes used to produce niobium capacitors are essentially the same as for tantalum capacitors. However, a price rise for tantalum in 2000/2001 encouraged the development of niobium electrolytic capacitors with manganese dioxide electrolyte, as well as polymer electrolyte which have been available since 2002.[4][13]
Electrical characteristics
[edit]The characteristics of electrolytic capacitors have certain characteristics that distinguish them from other types of capacitor like ceramic capacitors and film capacitors.
Series-equivalent circuit
[edit]
Niobium electrolytic capacitors as discrete components are not ideal capacitors, they have losses and parasitic inductive parts. All properties can be defined and specified by a series equivalent circuit composed out of an idealized capacitance and additional electrical components which model all losses and inductive parameters of a capacitor. In this series-equivalent circuit the electrical characteristics are defined by:
- C, the capacitance of the capacitor
- Rleak, the resistance representing the leakage current of the capacitor
- RESR, the equivalent series resistance which summarizes all ohmic losses of the capacitor, usually abbreviated as "ESR"
- LESL, the equivalent series inductance which is the effective self-inductance of the capacitor, usually abbreviated as "ESL".
Using a series equivalent circuit instead of a parallel equivalent circuit is specified by IEC/EN 60384-1.
Capacitance standard values and tolerances
[edit]The electrical characteristics of niobium electrolytic capacitors depend on structure of the anode and used electrolyte. This influences the capacitance value of the capacitors which depends on measuring frequency and temperature. The basic unit of electrolytic capacitors capacitance is microfarad (μF).
The capacitance value specified in the data sheets of the manufacturers is called rated capacitance CR or nominal capacitance CN and is the value for which the capacitor has been designed. Standardized measuring condition for electrolytic capacitors is an AC measuring method with a frequency of 100/120 Hz. The AC measuring voltage shall not exceed 0,5 V AC-RMS.
The percentage of allowed deviation of the measured capacitance from the rated value is called capacitance tolerance. Electrolytic capacitors are available in different tolerance series, whose values are specified in the E series specified in IEC 60063. For abbreviated marking in tight spaces, a letter code for each tolerance is specified in IEC 60062.
- rated capacitance, series E3, tolerance ±20%, letter code "M“
- rated capacitance, series E6, tolerance ±20%, letter code "M“
- rated capacitance, series E12, tolerance ±10%, letter code "K“
Rated and category voltage
[edit]
Referring to IEC/EN 60384-1 standard the allowed operating voltage for niobium capacitors is called "rated voltage UR " or "nominal voltage UN". The rated voltage UR is the maximum DC voltage or peak pulse voltage that may be applied continuously at any temperature within the rated temperature range TR (IEC/EN 60384-1).
The voltage proof of electrolytic capacitors decreases with increasing temperature. For some applications it is important to use a higher temperature range. Lowering the voltage applied at a higher temperature maintains safety margins. For some capacitor types therefore the IEC standard specify a "temperature derated voltage" for a higher temperature, the "category voltage UC". The category voltage is the maximum DC voltage or peak pulse voltage that may be applied continuously to a capacitor at any temperature within the category temperature range TC. The relation between both voltages and temperatures is given in the picture right.
Lower voltage applied may have positive influences for tantalum electrolytic capacitors. Lowering the voltage applied increases the reliability and reduce the expected failure rate.[14]
Applying a higher voltage than specified may destroy electrolytic capacitors.
Surge Voltage
[edit]The surge voltage indicates the maximum peak voltage value that may be applied to electrolytic capacitors during their application for a limited number of cycles. The surge voltage is standardized in IEC/EN 60384-1. For niobium electrolytic capacitors the surge voltage shall be not higher than round 1.3 times of the rated voltage, rounded off to the nearest volt. The surge voltage applied to niobium capacitors may influence the capacitors failure rate.
Reverse voltage
[edit]Niobium electrolytic are polarized and generally require anode electrode voltage to be positive relative to the cathode voltage.
Impedance, ESR and dissipation factor, ripple current , leakage current
[edit]General information to impedance, ESR, dissipation factor tan δ, ripple current, and leakage current see electrolytic capacitor
Reliability and life time
[edit]Reliability (failure rate]
[edit]General information to reliability and failure rate see electrolytic capacitor
Life time
[edit]The life timelife time, service life, load life or useful life of electrolytic capacitors is a special characteristic of non-solid electrolytic capacitors, especially non-solid aluminum electrolytic capacitors which liquid electrolyte can evaporate over the time leading to wear-out failures. Solid niobium capacitors with manganese dioxide electrolyte have no wear-out mechanism so that the constant failure rate least up to the point all capacitors have failed. They don’t have a life time specification like non-solid aluminum electrolytic capacitors.
However, solid polymer niobium electrolytic capacitors do have a life time specification. The polymer electrolyte have a small deterioration of conductivity by a thermal degradation mechanism of the conductive polymer. The electrical conductivity decreased, as a function of time, in agreement with a granular metal type structure, in which aging is due to the shrinking of the conductive polymer grains.[15] The life time of polymer electrolytic capacitors is specified in similar terms like non-solid e-caps but it’s life time calculation follows other rules leading to much longer operational life times.[16][17][18]
Failure modes, self-healing mechanism and application rules
[edit]The different types of electrolytic capacitors show different behaviors in long-term stability, inherent failure modes and their self-healing mechanisms. Application rules for types with an inherent failure mode are specified to ensure capacitors high reliability and long life.
| Type of electrolytic capacitors |
Long-term electrical behavior |
Failure modes | Self-healing mechanism |
Application rules |
|---|---|---|---|---|
| Tantalum e-caps solid MnO2 electrolyte |
stable | Field crystallization [19][20] |
Thermally induced insulating of faults in the dielectric by oxidization of the electrolyte MnO2 into insulating MnO2O3 if current availability is limited |
Voltage derating 50 % Series resistance 3 Ω/V [21][22] |
| Tantalum e-caps solid polymer electrolyte |
Deterioration of conductivity, ESR increases |
Field crystallization [19][20] |
Insulating of faults in the dielectric by oxidation or evaporation of the polymer electrolyte |
Voltage derating 20 % [21][22] |
| Niobium e-caps, solid MnO2 electrolyte |
stable | no unique determinable |
Thermally induced insulation of faults in the dielectric by oxidation of Nb2O5 into insulating NbO2 |
niobium anode: voltage derating 50 % niobium oxide anode: voltage derating 20 % [21][22] |
| Niobium e-caps solid polymer electrolyte |
Deterioration of conductivity, ESR increases |
no unique determinable |
Insulating of faults in the dielectric by oxidation or evaporation of the polymer electrolyte |
niobium anode: voltage derating 50 % niobium oxide anode: voltage derating 20 % [21][22] |

A rare failure in solid electrolytic capacitors is breakdown of the dielectric caused by faults or impurities. In niobium electrolytic capacitors the dielectric is niobium pentoxide (Nb2O5). Besides this pentoxide there is an additional niobium suboxide, niobium dioxde (NbO2). The NbO2 is a semi-conducting material with a higher conductivity than Nb2O5 but much lower than a short. In case of faults or impurities in the dielectric which evokes a partial dielectric breakdown the conducting channel. would be effectively isolated by oxidation of Nb2O5 into high ohmic NbO2 if energy is limited.
As more energy is applied to a faulty solid niobium eventually either the high ohmic NbO2 channel or the Nb2O5 dielectric breaks down and the capacitor exhibits a thermal runaway failure. In comparison to solid tantalum capacitors the thermal runaway of niobium anodes will occur at about three times higher power than of tantalum anodes. This gives a significant reduction (95%) of the ignition failure mode compared to solid tantalum capacitors.
The dielectric layer Nb2O5 of solid niobium electrolytic capacitors has a lower breakdown voltage proof than Ta2O5 in tantalum capacitors and therefore grows thicker per applied volt and so operates at lower field strength for a given voltage rating with the lower electrical stress the dielectric. In combination with niobium oxide anodes, which are more stable against oxygen diffusion that results in lower voltage derating rules compared with passivated niobium or tantalum anodes[3].
Niobium capacitors with solid conducting polymer electrolyte have a self-healing capability that eliminates thermal runaway failures. The repair takes place in response to the joule heating that occurs when a dielectric defect triggers a short circuit. The heating breaks the molecular chain of the conductive polymer near the defect, driving up its resistance and effectively forming a barrier against any current leaking from the electrode[23].
Additional information
[edit]Capacitor symbols
[edit]Electrolytic capacitor symbols
| Electrolytic capacitor |
Electrolytic capacitor |
Electrolytic capacitor |
Polarity marking
[edit]
Niobium capacitors are in general polarized components, with distinctly marked positive terminals. When subjected to reversed polarity (even briefly), the capacitor depolarizes and the dielectric oxide layer breaks down, which can cause it to fail even when later operated with correct polarity. If the failure is a short circuit (the most common occurrence), and current is not limited to a safe value, catastrophic thermal runaway may occur.
Standardization
[edit]The standardization for all electrical, electronic components and related technologies follows the rules given by the International Electrotechnical Commission (IEC),[24] a non-profit, non-governmental international standards organization. [25][26] The definition of the characteristics and the procedure of the test methods for capacitors for use in electronic equipment are set out in the generic specification:
- IEC 60384-1, Fixed capacitors for use in electronic equipment - Part 1: Generic specification
Until now (2014) no IEC detail specification for niobium electrolytic capacitors is available.
For electronics manufacturers in the United States the EIA publish a standard for niobium and tantalum chip capacitors:
- EIA-717-A Surface Mount Niobium and Tantalum Capacitor Qualification Specification
Features and Applications
[edit]- Niobium capacitors serve as a replacement for tantalum capacitors
- Niobium capacitors are available in SMD style, that makes them suitable for all portable electronic systems with flat design
- Niobium capacitors have no inrush current limitation
- Niobium capacitors are available with solid electrolyte for low ESR applications and stable electrical parameters
- Niobium capacitors have a limited number of manufacturers (AVX, Vishay, HolyStone)[27]
See also
[edit]- Capacitor
- Electrolytic capacitor
- Aluminum electrolytic capacitor
- Tantalum capacitor
- Polymer capacitor
- Types of capacitor
Bibliography
[edit]- R. P. Deshpande, Capacitors: Technology and Trends, ISBN 10: 1259007316ISBN 13: 9781259007316 [27]
- D. Bach, Dissertation, 05.06.2009, Universität Karlsruhe (TH), EELS investigations of stoichiometric niobium oxides and niobium-based capacitors [28]
- Ch. Schnitter: The taming of niobium. In: Bayer research, Bayer AG, 2004 (Version vom 11. Februar 2007 im Internet Archive), [29]
- Niobium Powder for Electrolytic Capacitor, JFE TECHNICAL REPORT No. 6 (Oct. 2005) PDF
References
[edit]- ^ a b J. Moore, Kemet, Nb capacitors compared to Ta capacitors a less costly alternative PDF
- ^ Niobium Powder for Electrolytic Capacitor, JFE TECHNICAL REPORT No. 6 (Oct. 2005) PDF
- ^ a b c T. Kárník, AVX, NIOBIUM OXIDE FOR CAPACITOR MANUFACTURING , METAL 2008, 13. –15. 5. 2008, PDF
- ^ a b T. Zednicek, S. Sita, C. McCracken, W. A. Millman, J. Gill, AVX, Niobium Oxide Technology Roadmap, CARTS 2002 PDF
- ^ Y. Pozdeev-Freeman, P. Maden, Vishay, Solid-Electrolyte Niobium Capacitors Exhibit Similar Performance to Tantalum, Feb 1, 2002, [1]
- ^ Rutronik, Tantalum & Niobium Capacitors, Technical Standards and Benefits PDF
- ^ Charles Pollack: D.R.P. 92564, filed 14. January 1896, granted 19. May 1897 D.R.P. 92564
- ^ D. F. Tailor, Tantalum and Tantalum Compounds, Fansteel Inc., Encyclopedia of Chemical Technology, Vol. 19, 2nd ed. 1969 John Wiley & sons, Inc.
- ^ R. L. Taylor and H. E. Haring, “A metal semi-conductor capacitor,” J. Electrochem. Soc., vol. 103, p. 611, November, 1956.
- ^ E. K. Reed, Jet Propulsion Laboratory, Characterization of Tantalum Polymer Capacitors, NEPP Task 1.21.5, Phase 1, FY05] [2]
- ^ D. A. McLean, F. S. Power, Proc. Inst. Radio Engrs. 44 (1956) 872
- ^ Tantalum-Niobium International Study Center, Tantalum and Niobium - Early History [3] and Applications for Niobium [4]
- ^ Ch. Schnitter, A. Michaelis, U. Merker, H.C. Starck, Bayer, New Niobium Based Materials for Solid Electrolyte Capacitors, Carts 2002
- ^ Ch. Reynolds, AVX, Technical Information, Reliability Management of Tantalum Capacitors, PDF
- ^ E. Vitoratos, S. Sakkopoulos, E. Dalas, N. Paliatsas, D. Karageorgopoulos, F. Petraki, S. Kennou, S.A. Choulis, Thermal degradation mechanisms of PEDOT:PSS, Organic Electronics, Volume 10, Issue 1, February 2009, Pages 61–66, [5]
- ^ Nichicon, Technical Guide, Calculation Formula of Lifetime PDF
- ^ Estimating of Lifetime FUJITSU MEDIA DEVICES LIMITED PDF
- ^ NIC Technical Guide, Calculation Formula of Lifetime
- ^ a b T.Zednicek, AVX, A Study of Field Crystallization in Tantalum Capacitors and its effect on DCL and Reliability, [6]
- ^ a b VISHAY, DC LEAKAGE FAILURE MODE, PDF
- ^ a b c d J.Gill, T. Zednicek, AVX, VOLTAGE DERATING RULES FOR SOLID TANTALUM AND NIOBIUM CAPACITORS, [7]
- ^ a b c d R. Faltus, AVX, Advanced capacitors ensure long-term control-circuit stability, 7/2/2012, EDT [8]
- ^ T. Zednicek, S. Zednicek, S. Sita, C. McCracken, W. A. Millman, AVX, Low ESR and Low Profile Technology on Niobium Oxide, AVX 2003 PDF
- ^ IEC Homepage [9]
- ^ IEC Webstore [10]
- ^ IEC/EN/DIN Standards, Beuth-Verlag
- ^ G. Roos, Digi-Key, Niobium Capacitors Slow to Take Hold, 2012-11-20, [11]
Polymer capacitor
[edit]
A polymer capacitor or more precisely a solid polymer electrolytic capacitor is a electrolytic capacitor with conductive polymer as solid electrolyte. They are polarized capacitors which anode electrode is made of a special metal on which by anodically oxidation (forming) an insulating oxide layer originates, which acts as the dielectric. The conductive polymer which covers the surface of the oxide layer serves as second electrode (cathode) of the capacitor. Depending on the used nature of the anode metal the family of solid polymer electrolytic capacitors is divided into three types:
- Polymer aluminum electrolytic capacitors,
- Polymer tantalum electrolytic capacitors and
- Polymer niobium electrolytic capacitors
All polymer capacitors are characterized by very low ESR values and high ripple current ratings. They are also characterized by low temperature depending of capacitance, impedance and ESR and also having a much longer life than non-solid (wet) electrolytic capacitors. Disadvantage is the higher leakage current compared with wet e-caps. Polymer capacitors are mainly used in electronic devices with flat or compact design as bypass or smoothing capacitors behind DC-DC converters for noise suppression on power lines.
Relatively new are hybrid polymer capacitors. This are polymer aluminum electrolytic capacitors in which a non-solid electrolyte bridge the gap between the polymer covering the porous anode foil and a so-called cathode foil. They combine low ESR and low leakage current and have lower prices.
Polymer capacitors are polarized components by the manufacturing principle and may only be operated with DC voltage. Voltages with reverse polarity, voltage or ripple current higher than specified can destroy the dielectric and thus the capacitor. A possible ripple voltage must not cause reversal. The destruction of electrolytic capacitors can have catastrophic consequences (explosion, fire).
Basic informations
[edit]Basic principle
[edit]
Polymer capacitors are in principle electrolytic capacitors. This capacitors are using a chemical feature of some special metals, earlier called “valve metals”, on which by anodically oxidation an insulating oxide layer serves as dielectric originates.
Main difference of the three types of polymer electrolytic capacitors is the used anode material and their oxide as dielectric:
- Polymer aluminum electrolytic capacitors use a high purity etched aluminum foil with aluminum oxide as dielectric
- Polymer tantalum electrolytic capacitors use a sintered pellet (“slug”) out of high purity tantalum powder with tantalum pentoxide as dielectric
- Polymer niobium electrolytic capacitors use a sintered “slug” out of high purity niobium or niobium oxide powder with niobium pentoxide as dielectric.
All anode materials are either etched or sintered and have a rough surface structure with a much higher surface compared to a smooth surface of the same area or the same volume. Applying a positive voltage to the above mentioned anode material in an electrolytic bath an oxide barrier layer with a thickness corresponding to the applied voltage will be formed (formation). This oxide layer acts as dielectric in an electrolytic capacitor. The properties of this oxide layers are given in the following table:
| Anode- material |
Dielectric | Permittivity ε |
Oxide structure |
Breakdown voltage (V/µm) |
Voltage proof (nm/V) |
|---|---|---|---|---|---|
| Aluminum | Aluminum oxide Al2O3 | 9.6 | amorphous | 710 | 1.4 |
| crystalline | 1000 | 1.0 | |||
| Tantalum | Tantalum pentoxide Ta2O5 | 27 | amorphous | 625 | 1.6 |
| Niobium or Niobium oxide |
Niobium pentoxide Nb2O5 | 41 | amorphous | 400 | 2.5 |
After forming a dielectric oxide on the rough anode structure a counter-electrode has to match the rough insulating oxide surface. This will be done by the conductive polymer electrolyte which acts as cathode electrode of the solid polymer electrolytic capacitor. This electrolyte can cover the rough anode structures by polymerization from a liquid solution into the solid polymerized structure.

Every electrolytic capacitor in principle forms a "plate capacitor" whose capacitance is greater, the larger the electrode area A and the permittivity ε are and the thinner the thickness (d) of the dielectric is.
The dielectric thickness of electrolytic capacitors is very thin in the range of nano-meter per volt. Otherwise the voltage strengths of these oxide layers are quite high. With this very thin dielectric oxide layer combined with a sufficient high dielectric strength the electrolytic capacitors can already achieve a high volumetric capacitance. This is one reason for the high capacitance values of electrolytic capacitors compared to conventional capacitors. All etched or sintered anodes have a much higher surface compared to a smooth surface of the same area or the same volume. That increases the later capacitance value, depending on the rated voltage, by the factor of up to 200 for non-solid aluminum electrolytic capacitors as well as for solid tantalum electrolytic capacitors
The large surface compared to a smooth one is the second reason for the relatively high capacitance values of electrolytic capacitors compared with other capacitor families.
All etched or sintered anodes have a much higher surface compared to a smooth surface of the same area or the same volume. That increases the later capacitance value, depending on the rated voltage by the factor of up to 200 for aluminum anodes as well as for sintered tantalum anodes[3][4][5]. The large surface compared to a smooth one is the second reason for the relatively high capacitance values of electrolytic capacitors compared with other capacitor families.
One special advantage is given for all electrolytic capacitors. Because the forming voltage defines the oxide layer thickness the voltage proof of the later electrolytic capacitor can be produced very simple for the desired rated value. Therefore the volume of a capacitor is defined by the product of capacitance and voltage, the so-called “CV-Volume”.
However, comparing the permittivities of the different oxide materials it is seen that tantalum pentoxide has an approximately 3 times higher permittivity than aluminum oxide. Tantalum electrolytic capacitors of a given CV value therefore are smaller than aluminum electrolytic capacitors. Comparing the characteristics of tantalum and niobium dielectric layers all values rounded leads to similar CV-volumes.
History
[edit]The phenomenon that can form an oxide layer on aluminum and other metals like tantalum, niobium, manganese, titanium,zinc, cadmium etc. in an electrochemical process, which block an electric current to flow in one direction but allow to flow in the other direction, however, it was discovered in 1875 by the French researcher and founder Eugène Ducretet. He coined for this kind of metals the term „valve metal“.
Charles Pollak (born Karol Pollak), a producer of accumulators, found out, that that the oxide layer on an aluminum anode remained stable in a neutral or alkaline electrolyte, even when the power was switched off. In 1896 he handed out this idea of an “Electric liquid capacitor with aluminum electrodes” as a patent of using the oxide layer in a polarized capacitor in combination with a neutral or slightly alkaline electrolyte. [6] The first industrial realized electrolytic capacitors in the 1930s and the following decades were aluminum electrolytic capacitors used mainly in radios.
The first tantalum electrolytic capacitors were developed in 1930 by Fansteel Metallurgical Corporation for military purposes [7] They adopt the basic construction of a wound cell and used a tantalum anode foil together with a tantalum cathode foil separated with a paper spacer impregnated with a liquid electrolyte. The relevant development of solid electrolyte tantalum capacitors began some years after William Shockley, John Bardeen and Walter Houser Brattain invented the transistor 1947. It was invented by Bell Laboratories in the early 1950s as a miniaturized, more reliable low-voltage support capacitor to complement their newly invented transistor. The solution R. L. Taylor and H. E. Haring from the Bell labs found in early 1950 was based on experiences with ceramics. They grind down tantalum to a powder, pressed this powder into a cylindrical form and then sintered the powder particles at high temperature between 1500 and 2000 °C under vacuum conditions to a pellet (“slug”). [8][9] These first sintered tantalum capacitors used a non-solid electrolyte, what don’t fit the concept of solid electronics. 1952 a targeted search in the Bell Labs for a solid electrolyte by D. A. McLean and F. S. Power led to the invention of manganese dioxide as a solid electrolyte for a sintered tantalum capacitor.[10]
With the beginning of the digitalization, 1971 launched Intel his first microcomputer MCS 4 and 1972 Hewlett Packard one of the first pocket calculator HP 35 [11][12] the requirements for capacitors increases in terms of lower losses. The equivalent series resistance (ESR) for bypass and decoupling capacitors of standard electrolytic capacitors should be decreases. [13]

It was not until 1983, by Sanyo with its "OS-CON" aluminum electrolytic capacitors, a new step of ESR reduction was developed. These capacitors are used an solid organic conductor, the charge transfer salt TTF-TCNQ (tetracyanoquinodimethane), which provided an improvement in conductivity by a factor of 10 with respect to the manganese dioxide electrolyte.[14] [15][16] The OS-CON TCNQ e-caps were in the years 1980s, 1990s very successful. However, 2010 Sanyo sold their OS-Con business to Panasonic [17]. Panasonic keep the trade name OS-CON but change the electrolyte. Now all OS-CON capacitors use a conductive polymer electrolyte. [18][19]
The next step in ESR reduction takes place with the development of conducting polymers by Alan J. Heeger, Alan MacDiarmid and Hideki Shirakawa in 1975. [20] The conductivity of conductive polymers such as polypyrrole (PPy) [21] or PEDOT [22] are by a factor of 100 to 500 better than that of TCNQ, and are close to the conductivity of metals.
In 1991 Panasonic with its "SP-Cap" [23] called polymer aluminum electrolytic capacitors came on the market. These aluminum electrolytic capacitors with polymer electrolytes reached very low ESR values direct comparable to ceramic multilayer capacitors (MLCCs). They were still less expensive than tantalum capacitors and with their flat design for laptops and cell phones a competition to tantalum chip capacitors, too..
Tantalum electrolytic capacitors with PPy polymer electrolyte cathode followed three years later. In 1993 NEC introduced his SMD polymer tantalum electrolytic capacitors called "NeoCap". 1997 followed Sanyo with the "POSCAP" polymer tantalum chips.
A new conductive polymer for tantalum polymer capacitors was presented by Kemet at the "1999 Carts" conference. [24] This capacitors used the new developed organic conductive polymer PEDT Poly(3,4-ethylenedioxythiophene), also known as PEDOT (trade name Baytron®) [25]
A price explosion for tantalum in 2000/2001 forces the new development of niobium electrolytic capacitors. Niobium is a sister metal to tantalum and serves as valve metal and generates an oxide layer during anodic oxidation. Niobium as raw material is much more abundant in nature than tantalum and is less expensive. The materials and processes used to produce niobium-dielectric capacitors are essentially the same as for existing tantalum-dielectric capacitors. The characteristics of this niobium electrolytic capacitors and tantalum electrolytic capacitors are roughly comparable. [26]. Niobium electrolytic capacitors with polymer electrolyte are available since 2002. [27][28]
Comparison of electrical parameters of polymer capacitor types
[edit]In order to compare the different characteristics of the different electrolytic capacitor types, capacitors with the same dimensions and of similar capacitance and voltage are compared in the following table. In such a comparison the values for ESR and permissible ripple current are the most important parameters. The lower the ESR, the higher the allowed ripple current per volume and better functionality of the capacitor in the circuit. However, better electrical parameters are combined with higher price.
| Electrolytic capacitor family |
Type 1) | Dimension DxL, WxHxL (mm) |
Max. ESR 100 kHz, 20 °C (mΩ) |
Max. Ripple current 85/105 °C (mA) |
Max. Leakage current after 2 Min. 2) (µA) |
|---|---|---|---|---|---|
| Aluminum-e-caps Polymer electrolyte |
Panasonic SP-UE 180/6.3 |
7.3x4.3x4.2 | 7 | 3700 | 100 (0.1CV) |
| Aluminum-e-caps Polymer electrolyte |
Kemet A700 100/10 |
7.3x4.3x4.0 | 10 | 4700 | 40 (0.04CV) |
| Aluminum-caps Polymer electrolyte |
Pan,OS-CON SVP 120/6.3 |
6.3x6 | 17 | 2780 | 200 (0.2CV) |
| Hybrid aluminum-caps, Polymer + non-solid electrolyte |
Pan,OS-CON ZA 100/25 |
6.3x8 | 30 | 2000 | 10 (0.01CV) |
| Tantalum-e-caps Polymer electrolyte |
Kemet T543 330/10 |
7.3x4.3x4,0 | 10 | 4900 | 100 (0.1CV) |
| Tantalum-e-caps Multianode, polymer |
Kemet T530 150/10 |
7.3x4.3x4.0 | 5 | 4970 | 100 (0.1CV) |
| Niobium-e-caps Polymer electrolyte |
NEC, NMC 100/10 |
7.3x4.3x2.8 | - | - | 20 (0.02CV) |
1) 100 µF/10 V, unless otherwise specified,
2) calculated for a capacitor 100 µF/10 V,
Capacitor symbols
[edit]Electrolytic capacitor symbols
| Electrolytic capacitor |
Electrolytic capacitor |
Electrolytic capacitor |
References
[edit]- ^ J.L. Stevens, A.C. Geiculescu, T.F. Strange, Dielectric Aluminum Oxides: Nano-Structural Features and Composites PDF
- ^ T. Kárník, AVX, NIOBIUM OXIDE FOR CAPACITOR MANUFACTURING , METAL 2008, 13. –15. 5. 2008, PDF
- ^ A. Albertsen, Jianghai Europe, Keep your distance – Voltage Proof of Electrolytic Capacitors, PDF
- ^ KDK, Specifications for Etched Foil for Anode, Low Voltage [12]
- ^ I.Horacek, T.Zednicek, S.Zednicek, T.Karnik, J.Petrzilek, P.Jacisko, P.Gregorova, AVX, High CV Tantalum Capacitors - Challenges and Limitations [13]
- ^ Charles Pollack: D.R.P. 92564, filed 14. Januar 1896, granted 19. Mai 1897 D.R.P. 92564
- ^ D. F. Tailor, Tantalum and Tantalum Compounds, Fansteel Inc., Encyclopedia of Chemical Technology, Vol. 19, 2nd ed. 1969 John Wiley & sons, Inc.
- ^ R. L. Taylor and H. E. Haring, “A metal semi-conductor capacitor,” J. Electrochem. Soc., vol. 103, p. 611, November, 1956.
- ^ E. K. Reed, Jet Propulsion Laboratory, Characterization of Tantalum Polymer Capacitors, NEPP Task 1.21.5, Phase 1, FY05] [14]
- ^ D. A. McLean, F. S. Power, Proc. Inst. Radio Engrs. 44 (1956) 872
- ^ Computerposter, [15]
- ^ K. Lischka, Spiegel 27.09.2007, 40 Jahre Elektro-Addierer: Der erste Taschenrechner wog 1,5 Kilo, [16]
- ^ Larry E. Mosley, Intel Corporation, Capacitor Impedance Needs For Future Microprocessors, CARTS USA 2006, [17]
- ^ Shinichi Niwa, Yutaka Taketani, Development of new series of aluminium solid capacitors with organic Semiconductive electrolyte (OS-CON), Journal of Power Sources, Volume 60, Issue 2, June 1996, Pages 165–171, [18]
- ^ Kuch, Investigation of charge transfer complexes:TCNQ-TTF, [19]
- ^ Sanyo, OS-CON, Technical Book Ver. 15, 2007 [20]
- ^ Panasonic Announces that it Makes SANYO its Wholly-owned Subsidiary through Share Exchange PDF
- ^ New OS-CON Capacitors, Aluminum-Polymer Solid Capacitors; Panasonic [21]
- ^ Panasonic, Basic structure of OS-CON PDF
- ^ About the Nobel Prize in Chemistry 2000, Advanced Information, October 10, 2000,[22]
- ^ Y. K. ZHANG, J. LIN,Y. CHEN, Polymer Aluminum Electrolytic Capacitors with Chemically-Polymerized Polypyrrole (PPy) as Cathode Materials Part I. Effect of Monomer Concentration and Oxidant on Electrical Properties of the Capacitors, PDF
- ^ U. Merker, K. Wussow, W. Lövenich, H. C. Starck GmbH, New Conducting Polymer Dispersions for Solid Electrolyte Capacitors, PDF
- ^ Panasonic, SP-Caps [23]
- ^ John Prymak, Kemet, Replacing MnO2 with Polymers, 1999 CARTS [24]
- ^ F. Jonas, H.C.Starck, Baytron, Basic chemical and physical properties, Präsentation 2003, [www.hcstarck.de]
- ^ Y. Pozdeev-Freeman, P. Maden, Vishay, Solid-Electrolyte Niobium Capacitors Exhibit Similar Performance to Tantalum, Feb 1, 2002, [25]
- ^ Ch. Schnitter, A. Michaelis, U. Merker, H.C. Starck, Bayer, New Niobium Based Materials for Solid Electrolyte Capacitors, Carts 2002
- ^ T. Zednicek, S. Sita, C. McCracken, W. A. Millman, J. Gill, AVX, Niobium Oxide Technology Roadmap, CARTS 2002 [26]