Dihalomethane: Difference between revisions
Content deleted Content added
No edit summary |
Translating from German verison, de:Special:Diff/212444986 |
||
| Line 11: | Line 11: | ||
* [[Chloroiodomethane]] |
* [[Chloroiodomethane]] |
||
* [[Fluoroiodomethane]] |
* [[Fluoroiodomethane]] |
||
{| class="wikitable float-right" style="text-align:center; font-size:90%;" |
|||
|-- |
|||
| [[Structural Formula]] |
|||
| [[File:Difluoromethane Structural Formula V1.svg|100px]] |
|||
| [[File:Dichloromethane Structural Formula V1.svg|100px]] |
|||
| [[File:Dibromomethane Structural Formula V1.svg|100px]] |
|||
| [[File:Diiodomethane Structural Formula V1.svg|100px]] |
|||
|-- |
|||
| Name |
|||
| [[Difluoromethane]] || [[Dichloromethane]] || [[Dibromoethane]] || [[Diiodomethane]] |
|||
|-- |
|||
| [[Melting point]] |
|||
| −136 [[Degree Celsius|°C]]<ref name="GESTIS_F">{{GESTIS|ZVG=100470|CAS=75-10-5|Name=Difluormethan|Date=2020-02-29}}</ref> |
|||
| −97 °C<ref name="GESTIS_CL">{{GESTIS|Name=Dichlormethan|ZVG=12630|CAS=75-09-2|Date=2020-02-29}}</ref> |
|||
| −52 °C<ref name="GESTIS_BR">{{GESTIS|Name=Dibrommethan|ZVG=30900|CAS=74-95-3|Date=2020-02-29}}</ref> |
|||
| 6 °C<ref name="GESTIS_I">{{GESTIS|Name=Methyleniodid|ZVG=492386|CAS=75-11-6|Date=2020-02-29}}</ref> |
|||
|-- |
|||
| [[Boiling point]] |
|||
| −51,7 °C<ref name="GESTIS_F"/> |
|||
| 40 °C<ref name="GESTIS_CL"/> |
|||
| 97 °C<ref name="GESTIS_BR"/> |
|||
| Zersetzung<ref name="GESTIS_I"/> |
|||
|-- |
|||
| [[Space-filling model]] |
|||
| [[File:Difluoromethane-3D-vdW.png|90px]] |
|||
| [[File:Dichloromethane-3D-vdW.png|110px]] |
|||
| [[File:Dibromomethane 3D.png|120px]] |
|||
| [[File:Diiodomethane-3D-vdW.png|130px]] |
|||
|} |
|||
== See also == |
== See also == |
||
Revision as of 10:00, 21 April 2024
You can help expand this article with text translated from the corresponding article in German. (March 2020) Click [show] for important translation instructions.
|
The dihalomethanes are organic compounds in which two hydrogen atoms in methane are replaced by halogen atoms. They belong to the haloalkanes, specifically the subgroup of halomethanes, and contains ten members.
There are four members with only one kind of halogen atom: difluoromethane, dichloromethane, dibromomethane and diiodomethane.
There are six members with two kinds of halogen atoms:
- Bromochloromethane
- Bromofluoromethane
- Bromoiodomethane
- Chlorofluoromethane
- Chloroiodomethane
- Fluoroiodomethane
| Structural Formula | 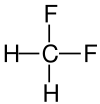
|

|
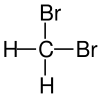
|

|
| Name | Difluoromethane | Dichloromethane | Dibromoethane | Diiodomethane |
| Melting point | −136 °C[1] | −97 °C[2] | −52 °C[3] | 6 °C[4] |
| Boiling point | −51,7 °C[1] | 40 °C[2] | 97 °C[3] | Zersetzung[4] |
| Space-filling model | 
|

|
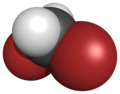
|

|
See also
Wikimedia Commons has media related to Dihalomethanes.
- ^ a b Record of Difluormethan in the GESTIS Substance Database of the Institute for Occupational Safety and Health, accessed on 2020-02-29.
- ^ a b Record of Dichlormethan in the GESTIS Substance Database of the Institute for Occupational Safety and Health, accessed on 2020-02-29.
- ^ a b Record of Dibrommethan in the GESTIS Substance Database of the Institute for Occupational Safety and Health, accessed on 2020-02-29.
- ^ a b Record of Methyleniodid in the GESTIS Substance Database of the Institute for Occupational Safety and Health, accessed on 2020-02-29.
