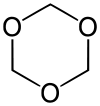
1,3,5-Trioxane
| |
| Names | |
|---|---|
| IUPAC name
1,3,5-Trioxane
| |
| Other names
s-Trioxane
1,3,5-Trioxacyclohexane Trioxymethylene Metaformaldehye Trioxin | |
| Identifiers | |
3D model (JSmol)
|
|
| ECHA InfoCard | 100.003.466 |
| RTECS number |
|
CompTox Dashboard (EPA)
|
|
| |
| Properties | |
| C3H6O3 | |
| Molar mass | 90.08 g/mol |
| Appearance | white crystalline solid |
| Density | 1.17 g/cm³ (65 °C) |
| Melting point | 64 °C |
| Boiling point | 114.5 °C |
| 17.2 g/100 ml (18 °C) | |
| Hazards | |
| NFPA 704 (fire diamond) | |
| Flash point | 45 °C |
| Related compounds | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
1,3,5-Trioxane, sometimes also called trioxin, is a chemical compound with molecular formula C3H6O3. It is a stable cyclic trimer of formaldehyde, and one of the two trioxane isomers; its molecular backbone consists of a six membered ring with three carbon atoms alternating with three oxygen atoms.
1,3,5-Trioxane is a white solid with a chloroform-like odor. It is a feedstock for certain types of plastic, an ingredient in some solid fuel tablet formulas, is used in chemical laboratories as a stable source of formaldehyde.
Uses
In chemistry, it is used as a stable, easily handled source of anhydrous formaldehyde. In acidic solutions, it decomposes to generate three molecules of formaldehyde. It may also be used in polymerization to form acetal resins, such as polyoxymethylene plastic.
Trioxane is combined with hexamine and compressed into solid bars to make hexamine fuel tablet, used by the military and outdoorsmen as a cooking fuel.
1,3,5-Trioxane is a mortician's restorative chemical that repairs cells and maintains the corpse's contours after postmortem tissue constriction.[citation needed]

