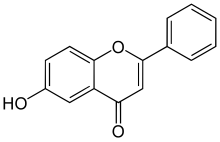
6-Hydroxyflavone
| |

| |
| Names | |
|---|---|
| IUPAC name
6-hydroxy-2-phenylchromen-4-one
| |
| Other names
6-Monohydroxyflavone; 6-Hydroxy-2-phenyl-4-benzopyrone
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.027.005 |
| EC Number |
|
| KEGG | |
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C15H10O3 | |
| Molar mass | 238.242 g·mol−1 |
| Melting point | 234 to 236 °C (453 to 457 °F; 507 to 509 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
6-Hydroxyflavone is a flavone, a type of chemical compound. It is one of the noncompetitive inhibitors of cytochrome P450 2C9. It is reported in leaves of Barleria prionitis Linn. (a common Acanthaceae from India).[1] 6-Hydroxyflavone may have a potential as a therapeutic drug capable for the treatment of anxiety-like disorders.[2]
References
- ^ Medicinal plants, Chemistry and properties by M Daniel.
- ^ "GABAA receptor subtype selectivity underlying anxiolytic effect of 6-hydroxyflavone". ScienceDirect. Retrieved 2011-06-12.
