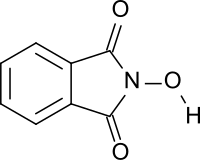
N-Hydroxyphthalimide
| |
| Names | |
|---|---|
| IUPAC name
2-Hydroxyisoindole-1,3-dione
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.007.600 |
| EC Number |
|
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C8H5NO3 | |
| Molar mass | 163.132 g·mol−1 |
| Hazards | |
| GHS labelling: | |

| |
| Warning | |
| H315, H319, H335 | |
| P261, P264, P271, P280, P302+P352, P304+P340, P305+P351+P338, P312, P321, P332+P313, P337+P313, P362, P403+P233, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
N-Hydroxyphthalimide is the N-hydroxy derivative of phthalimide. The compound is used, inter alia, as catalyst for oxidation reactions, in particular for the selective oxidation (e. g. alkanes to alcohols) with molecular oxygen under mild conditions.[1][2]
Occurrence and production
The synthesis of N-hydroxyphthalimide from phthaloyl chloride and hydroxylamine hydrochloride in the presence of sodium carbonate in aqueous solution was first reported by Lassar Cohn in 1880 (referred to as "Phthalylhydroxylamin").[3]
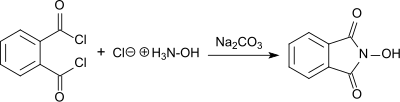
The product forms as a red sodium salt under basic conditions, while white N-hydroxyphthalimide precipitates in 55% yield as the solution is acidified. N-hydroxyphthalimide is also produced by reacting hydroxylamine hydrochloride with diethyl phthalate in the presence of sodium acetate,[4] or with phthalic anhydride in the presence of sodium carbonate with heating. In the last case, an overall yield of 76% is produced following purification by recrystallization.[5]
Microwave irradiation of phthalic anhydride and hydroxylamine hydrochloride in pyridine produces N-hydroxyphthalimide in 81% yield.[6] Even in the absenceof a base, phthalic anhydride and hydroxylamine phosphate react to produce N-hydroxyphthalimide in 86% yield when heated to 130 °C.[7]

Properties
N-Hydroxyphthalimide is a colorless to yellow, odorless crystalline powder which is soluble in water and organic solvents such as acetic acid, ethyl acetate and acetonitrile.[8] The compound exists in two different-colored monoclinic crystal forms. In case of the colorless white form, the N-OH group is rotated about 1.19° from the plane of the molecule, while in the yellow form it is much closer to planarity (0.06° rotation).[9]
The color of the synthesized N-hydroxyphthalimide depends on the type of solvent used; the color transition from white to yellow is irreversible.[10] N-hydroxyphthalimide forms strongly colored, mostly yellow or red salts with alkali and heavy metals, ammonia and amines.[11] Hydrolysis of N-hydroxyphthalimide by the addition of strong bases produces phthalic acid monohydroxamic acid by adding water across one of the carbon–nitrogen bonds.[4] N-hydroxyphthalimide ethers, on the other hand, are colorless and provide O-alkylhydroxylamines by alkaline hydrolysis or cleavage through hydrazine hydrate.
The "phthalylhydroxylamine" reported by Cohn was known to have a molecular formula of C
8H
5NO
3 but the exact structure was not known.[3] Three possibilities were discussed and are shown in the Figure below: a mono-oxime of phthalic anhydride ("phthaloxime", I), an expanded ring with two heteroatoms, (2,3-benzoxazine-1,4-dione, II), and N-hydroxyphthalimide (III).[10][12] It was not until the 1950s that Cohn's product was definitely shown to be (III), N-hydroxyphthalimide.[13]

8H
5NO
3 considered as Cohn's "phthalylhydroxylamine"
Applications and reactions
Nefkens and Tesser developed a technique for generating active esters from N-hydroxyphthalimide[14] for use in peptide synthesis,[15] an approach later extended to using N-hydroxysuccinimide.[16] The ester linkage is formed between the N-hydroxyphthalimide and a carboxylic acid by elimination of water, the coupling achieved with N,N'-dicyclohexylcarbodiimide (DCC). For peptide synthesis, the N-terminus of the growing peptide is protected with tert-butyloxycarbonyl while its C-terminus (Z-NH-CH(R)-COOH) is coupled to N-hydroxyphthalimide. An ester of the next amino acid in the desired peptide sequence is shaken with activated ester, adding to the chain and displacing the N-hydroxyphthalimide. This reaction is quantitative and nearly instantaneous at 0 °C.[15][17] The resulting ester needs to be hydrolysed before the cycle can be repeated.

The N-hydroxyphthalimide can be removed by shaking with sodium bicarbonate,[15] but the N-hydroxysuccinimide approach shows greater reactivity and convenience, and is generally preferred.[16][17]
Esters of N-hydroxyphthalimide and activated sulfonic acids such as trifluoromethanesulfonic anhydride or p-toluenesulfonyl chloride are used as so-called photoacids, which split off protons during UV irradiation.

The protons generated serve for the targeted local degradation of acid-sensitive photoresists.[18]
N-hydroxyphthalimide can be converted with vinyl acetate in the presence of palladium(II)acetate to the N-vinyloxyphthalimide, which is quantitatively hydrogenated to N-ethoxyphthalimide and after purified by cleavage, yielding O-ethylhydroxylamine.[19]

A variety of different functional groups can be oxidized with the aminoxyl radical (phthalimide-N-oxyl, PINO)[20] formed by the abstraction of a hydrogen atom from N-hydroxyphthalimide under gentle conditions (similar to TEMPO):[1]
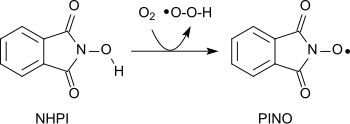
Using molecular oxygen alkanes can be oxidized to form alcohols, secondary alcohols to ketones, acetals to esters and alkenes to epoxides.[21][22][23] Amides can be converted into carbonyl compounds with N-hydroxyphthalimide and cobalt(II)salts under mild conditions.[24]

Efficient oxidation reactions of precursors of important basic chemicals are of particular technical interest. For example, ε-caprolactam can be prepared using NHPI from the so-called KA oil ("ketone-alcohol" oil, a mixture of cyclohexanol and cyclohexanone) which is obtained during the oxidation of cyclohexane. The reaction proceeds via cyclohexanol hydroperoxide which reacts with ammonia to give peroxydicyclohexylamine followed by a rearrangement in the presence of catalytic amounts of lithium chloride.[22][25]
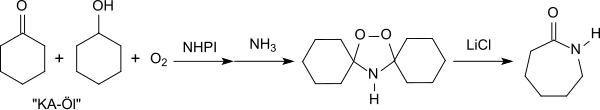
The use of N-hydroxyphthalimide as a catalyst in the oxidation of KA oil avoids the formation of the undesirable by-product ammonium sulfate which is produced by the conventional ε-caprolactam synthesis (Beckmann rearrangement of cyclohexanone oxime with sulfuric acid).
Alkanes are converted into nitroalkanes in the presence of nitrogen dioxide.[26]
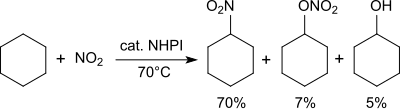
Cyclohexane is converted at 70 °C with nitrogen dioxide/air into a mixture of nitrocyclohexane (70%), cyclohexyl nitrate (7%) and cyclohexanol (5%).
Furthermore, applications of N-hydroxyphthalimide as oxidizing agents in photographic developers[27] and as charge control agents in toners[28] have been described in the patent literature.
Phthalimido-N-oxyl (PINO)
The radical derived by removal of an H-atom from N-hydroxyphthalimide is called N-phthalimido-N-oxyl, acronym being PINO. It is a powerful H-atom abstracting agent.[1] The bond dissociation energy of NHPI (i.e., PINO-H) is between 88-90 kcal/mol, depending on the solvent.[29]
References
- ^ a b c Recupero, Francesco; Punta, Carlo (2007). "Free Radical Functionalization of Organic Compounds Catalyzed by N-Hydroxyphthalimide". Chem. Rev. 107 (9): 3800–3842. doi:10.1021/cr040170k. PMID 17848093.
- ^ Melone, Lucio; Punta, Carlo (2013). "Metal-free aerobic oxidations mediated by N-hydroxyphthalimide. A concise review". Beilstein J. Org. Chem. 9: 1296–1310. doi:10.3762/bjoc.9.146. PMID 23843925.
- ^ a b Cohn, Lassar (1880). "Phthalylhydroxylamin: Ueberführung der Phthalsäure in Salicylsäure" [N-hydroxyphthalimide: Conversion of phthalic acid into salicylic acid]. Justus Liebigs Ann. Chem. (in German). 205 (3): 295–314. doi:10.1002/jlac.18802050304.
- ^ a b Bauer, Ludwig; Miarka, Stanley V. (1957). "The Chemistry of N-Hydroxyphthalimide". J. Am. Chem. Soc. 79 (8): 1983–1985. doi:10.1021/ja01565a061.
- ^ Gross, H.; Keitel, I. (1969). "Zur Darstellung von N-Hydroxyphthalimid und N-Hydroxysuccinimid" [On the preparation of N-hydroxyphthalimide and N-hydroxysuccinimide]. J. Prakt. Chem. (in German). 311 (4): 692–693. doi:10.1002/prac.19693110424.
- ^ Sugamoto, Kazuhiro; Matsushita, Yoh‐ichi; Kameda, Yu‐hei; Suzuki, Masahiko; Matsui, Takanao (2005). "Microwave‐assisted Synthesis of N‐Hydroxyphthalimide Derivatives". Synth. Commun. 35 (1): 67–70. doi:10.1081/SCC-200046498.
- ^ EP application 1085013, Elke Fritz-Langhals, "Verfahren zur Herstellung cyclischer N-Hydroxy-dicarboximide (Process for the preparation of cyclic N-hydroxydicarboximides)", published 2001-03-21, assigned to Consortium für elektrochemische Industrie GmbH
- ^ Gambarotti, Cristian; Punta, Carlo; Recupero, Francesco; Zlotorzynska, Maria; Sammis, Glenn (2013). "N-Hydroxyphthalimide". N-Hydrophthalimide. Encyclopedia of Reagents for Organic Synthesis. doi:10.1002/047084289X.rn00598.pub2. ISBN 978-0471936237.
- ^ Reichelt, Hendrik; Faunce, Chester A.; Paradies, Henrich H. (2007). "Elusive forms and structures of N-hydroxyphthalimide: The colorless and yellow crystal forms of N-hydroxyphthalimide". J. Phys. Chem. A. 111 (13): 2587–2601. doi:10.1021/jp068599y. PMID 17388355.
- ^ a b Ames, D. E.; Grey, T. F. (1955). "N-Hydroxy-imides. Part II. Derivatives of homophthalic and phthalic acid". J. Chem. Soc.: 3518–3521. doi:10.1039/JR9550003518.
- ^ Porcheddu, Andrea; Giacomelli, Giampaolo (2009). "Synthesis of oximes and hydroxamic acids". In Rappaport, Zvi; Lieberman, Joel F. (eds.). The Chemistry of Hydroxylamines, Oximes, and Hydroxamic Acids, Part 1. Chichester: Wiley. pp. 224–226. ISBN 978-0-470-51261-6.
- ^ Bradly, Oscar L.; Baker, Leslie C.; Goldstein, Richard F.; Harris, Samuel (1928). "LXVIII.—The isomerism of the oximes. Part XXXIII. The oximes of opianic acid and of phthalic anhydride". J. Chem. Soc.: 529–539. doi:10.1039/JR9280000529.
- ^ Hurd, Charles D.; Buess, Charles M.; Bauer, Ludwig (1954). "Succino- and phthalo-hydroxamic acids". J. Org. Chem. 19 (7): 1140–1149. doi:10.1021/jo01372a021.
- ^ Nefkens, G. H. L.; Tesser, G. I.; Nivard, R. J. F. (1962). "Synthesis and reactions of esters of N-hydroxyphthalimide and N-protected amino acids". Recl. Trav. Chim. Pays-Bas. 81 (8): 683–690. doi:10.1002/recl.19620810807.
- ^ a b c Nefkens, G. H. L.; Tesser, G. I. (1961). "A Novel Activated Ester in Peptide Synthesis". J. Am. Chem. Soc. 83 (5): 1263. doi:10.1021/ja01466a068.
- ^ a b Anderson, George W.; Zimmerman, Joan E.; Callahan, Francis M. (1964). "The Use of Esters of N-Hydroxysuccinimide in Peptide Synthesis". J. Am. Chem. Soc. 86 (9): 1839–1842. doi:10.1021/ja01063a037.
- ^ a b Bodanszky, Miklos (1993). "Activation and Coupling". Principles of Peptide Synthesis (2nd ed.). Springer-Verlag. pp. 9–61. doi:10.1007/978-3-642-78056-1_2. ISBN 9783642780561.
- ^ 0919867, K. Elian, E. Günther, R. Leuschner
- ^ 1995025090, D.M.C. Callant, A.M.C.F. Castelijns, J.G. De Vries
- ^ S. Coseri (2009), "Phthalimide‐N‐oxyl (PINO) Radical, a Powerful Catalytic Agent: Its Generation and Versatility Towards Various Organic Substrates", Catal. Rev. Sci. Eng., vol. 51, no. 2, pp. 218–292, doi:10.1080/01614940902743841
- ^ Y. Ishii, K. Nakayama, M. Takeno, S. Sakaguchi, T. Iwahama, Y. Nishiyama (1995), "Novel Catalysis by N-Hydroxyphthalimide in the Oxidation of Organic Substrates by Molecular Oxygen", J. Org. Chem., vol. 60, no. 13, pp. 3934–3935, doi:10.1021/jo00118a002
{{citation}}: CS1 maint: multiple names: authors list (link) - ^ a b "Discovery of a carbon radical producing catalyst and its application to organic synthesis" (PDF). TCIMAIL, Number 116. Tokyo Chemical Industry Co. Ltd. April 2003. Retrieved 2016-08-11.
- ^ B.B. Wentzel, M.P.J. Donners, P.L. Alsters, M.C. Feiters, R.J.M. Nolte (2000), "N-Hydroxyphthalimide/cobalt(II) catalyzed low temperature benzylic oxidation using molecular oxygen", Tetrahedron, vol. 56, no. 39, pp. 7797–7803, doi:10.1016/S0040-4020(00)00679-7
{{citation}}: CS1 maint: multiple names: authors list (link) - ^ F. Minisci, C. Punta, F. Recupero, F. Fontana, G.F. Pedulli (2002), "Aerobic Oxidation of N-Alkylamides Catalyzed by N-Hydroxyphthalimide under Mild Conditions. Polar and Enthalpic Effects", J. Org. Chem., vol. 67, no. 8, pp. 2671–2676, doi:10.1021/jo016398e, PMID 11950315
{{citation}}: CS1 maint: multiple names: authors list (link) - ^ O. Fukuda, S. Sakaguchi, Y. Ishii (2001), "A new strategy for catalytic Baeyer-Villiger oxidation of KA-oil with molecular oxygen using N-hydroxyphthalimide", Tetrahedron Lett., vol. 42, no. 20, pp. 3479–3481, doi:10.1016/S0040-4039(01)00469-5
{{citation}}: CS1 maint: multiple names: authors list (link) - ^ S. Sakaguchi, Y. Nishiwaki, T. Kitamura, Y. Ishii (2001), "Efficient catalytic alkane nitration with NO2 under air assisted by N-hydroxyphthalmide", Angew. Chem., Int. Edit., vol. 40, no. 1, pp. 222–224, doi:10.1002/1521-3773(20010105)40:1<222::AID-ANIE222>3.0.CO;2-W
{{citation}}: CS1 maint: multiple names: authors list (link) - ^ EP application 0664479, W. Ishikawa & T. Sampei, "Method of processing silver halide photographic lightsensitive material", published 1994-7-26, assigned to Konica Corp.
- ^ US 5332637, J.C. Wilson; S.M. Bonser & H.W. Osterhoudt, "Electrostatographic dry toner and developer compositions with hydroxyphthalimide", issued 1994-7-26, assigned to Eastman Kodak Co.
- ^ Coseri, Sergiu (2009). "Phthalimide‐N‐oxyl (PINO) Radical, a Powerful Catalytic Agent: Its Generation and Versatility Towards Various Organic Substrates". Catalysis Reviews. 51 (2): 218–292. doi:10.1080/01614940902743841.
