Primary ciliary dyskinesia
| Primary ciliary dyskinesia | |
|---|---|
| Other names | Immotile ciliary syndrome or Kartagener syndrome |
 | |
| Normal cilia (A) and cilia representative of Kartagener's syndrome (B) | |
| Specialty | Pulmonology |
Primary ciliary dyskinesia (PCD) is a rare, autosomal recessive genetic ciliopathy, that causes defects in the action of cilia lining the upper and lower respiratory tract, sinuses, Eustachian tube, middle ear, Fallopian tube, and flagella of sperm cells. The alternative name of "immotile ciliary syndrome" is no longer favored as the cilia do have movement, but are merely inefficient or unsynchronized. When accompanied by situs inversus the condition is known as Kartagener syndrome.[1]
Respiratory epithelial motile cilia, which resemble microscopic "hairs" (although structurally and biologically unrelated to hair), are complex organelles that beat synchronously in the respiratory tract, moving mucus toward the throat. Normally, cilia beat 7 to 22 times per second, and any impairment can result in poor mucociliary clearance, with subsequent upper and lower respiratory infection. Cilia also are involved in other biological processes (such as nitric oxide production), currently the subject of dozens of research efforts.[2]
Signs and symptoms
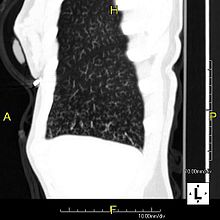


The main consequence of impaired ciliary function is reduced or absent mucus clearance from the lungs, and susceptibility to chronic recurrent respiratory infections, including sinusitis, bronchitis, pneumonia, and otitis media. Progressive damage to the respiratory system is common, including progressive bronchiectasis beginning in early childhood, and sinus disease (sometimes becoming severe in adults). However, diagnosis is often missed early in life despite the characteristic signs and symptoms.[3] In males, immotility of sperm can lead to infertility, although conception remains possible through the use of in vitro fertilization, there also are reported cases where sperm were able to move.[4] Trials have also shown that there is a marked reduction in fertility in females with Kartagener's syndrome due to dysfunction of the oviductal cilia.[5]
Many affected individuals experience hearing loss and show symptoms of otitis media which demonstrates variable responsiveness to the insertion of myringotomy tubes or grommets. Some patients have a poor sense of smell, which is believed to accompany high mucus production in the sinuses (although others report normal – or even acute – sensitivity to smell and taste). Clinical progression of the disease is variable, with lung transplantation required in severe cases. Susceptibility to infections can be drastically reduced by an early diagnosis. Treatment with various chest physiotherapy techniques has been observed to reduce the incidence of lung infection and to slow the progression of bronchiectasis dramatically. Aggressive treatment of sinus disease beginning at an early age is believed to slow long-term sinus damage (although this has not yet been adequately documented). Aggressive measures to enhance clearance of mucus, prevent respiratory infections, and treat bacterial superinfections have been observed to slow lung-disease progression. Although the true incidence of the disease is unknown, it is estimated to be 1 in 32,000,[6] although the actual incidence may be as high as 1 in 15,000.
Genetics
PCD is a genetically heterogeneous disorder affecting motile cilia[7] which are made up of approximately 250 proteins.[8] Around 90%[9] of individuals with PCD have ultrastructural defects affecting protein(s) in the outer and/or inner dynein arms, which give cilia their motility, with roughly 38%[9] of these defects caused by mutations on two genes, DNAI1 and DNAH5, both of which code for proteins found in the ciliary outer dynein arm.[citation needed]
There is an international effort to identify genes that code for inner dynein arm proteins or proteins from other ciliary structures (radial spokes, central apparatus, etc.) associated with PCD.[citation needed] The role of DNAH5 in heterotaxy syndromes and left-right asymmetry is also under investigation. At least 32 genes have been implicated in this condition.[10]
| Type | OMIM | Gene | Locus |
|---|---|---|---|
| CILD1 | 244400 | DNAI1 | 9p21-p13 |
| CILD2 | 606763 | ? | 19q13.3-qter |
| CILD3 | 608644 | DNAH5 | 5p |
| CILD4 | 608646 | ? | 15q13 |
| CILD5 | 608647 | ? | 16p12 |
| CILD6 | 610852 | TXNDC3 | 7p14-p13 |
| CILD7 | 611884 | DNAH11 | 7p21 |
| CILD8 | 612274 | ? | 15q24-q25 |
| CILD9 | 612444 | DNAI2 | 17q25 |
| CILD10 | 612518 | KTU | 14q21.3 |
| CILD11 | 612649 | RSPH4A | 6q22 |
| CILD12 | 612650 | RSPH9 | 6p21 |
| CILD13 | 613190 | LRRC50 | 16q24.1 |
Another gene associated with this condition is GAS2L2.[11]
Pathophysiology

This condition is genetically inherited. Structures that make up the cilia, including inner and/or outer dynein arms, central apparatus, radial spokes, etc. are missing or dysfunctional and thus the axoneme structure lacks the ability to move. Axonemes are the elongated structures that make up cilia and flagella. Additionally, there may be chemical defects that interfere with ciliary function in the presence of adequate structure. Whatever the underlying cause, dysfunction of the cilia begins during and impacts the embryologic phase of development.[citation needed]

Specialised monocilia known as nodal cilia are at the heart of this problem. They lack the central-pair microtubules of ordinary motile cilia and so rotate clockwise rather than beat; in the primitive node at the anterior end of the primitive streak in the embryo, these are angled posteriorly[12][13] such that they describe a D-shape rather than a circle.[13] This has been shown to generate a net leftward flow in mouse and chick embryos, and sweeps the protein to the left, triggering normal asymmetrical development.[citation needed]
However, in some individuals with PCD, mutations thought to be in the gene coding for the key structural protein left-right dynein (lrd)[7] result in monocilia which do not rotate. There is therefore no flow generated in the node, Shh moves at random within it, and 50% of those affected develop situs inversus, which can occur with or without dextrocardia, where the laterality of the internal organs is the mirror-image of normal. Affected individuals therefore have Kartagener syndrome. This is not the case with some PCD-related genetic mutations: at least 6%[citation needed] of the PCD population have a condition called situs ambiguus or heterotaxy, where organ placement or development is neither typical (situs solitus) nor totally reversed (situs inversus totalis) but is a hybrid of the two. Splenic abnormalities such as polysplenia, asplenia and complex congenital heart defects are more common in individuals with situs ambiguus and PCD, as they are in all individuals with situs ambiguus.[14]
The genetic forces linking failure of nodal cilia and situs issues and the relationship of those forces to PCD are the subject of intense research interest. However, knowledge in this area is constantly advancing.[citation needed]
Relation to other rare genetic disorders
Recent findings in genetic research have suggested that a large number of genetic disorders, both genetic syndromes and genetic diseases, that were not previously identified in the medical literature as related, may be, in fact, highly related in the genetypical root cause of the widely varying, phenotypically-observed disorders. Thus, PCD is a ciliopathy. Other known ciliopathies include Bardet–Biedl syndrome, polycystic kidney and liver disease, nephronophthisis, Alström syndrome, Meckel–Gruber syndrome and some forms of retinal degeneration.[15]
Diagnosis
Several diagnostic tests for this condition have been proposed.[16] These include nasal nitric oxide levels as a screening test, light microscopy of biopsies for ciliary beat pattern and frequency and electron microscopic examination of dynein arms, as the definite diagnosis method. Genetic testing has also been proposed but this is difficult given that there are multiple genes involved.[citation needed]
Classification

When accompanied by the combination of situs inversus (reversal of the internal organs), chronic sinusitis, and bronchiectasis, it is known as Kartagener syndrome[1] (only 50% of primary ciliary dyskinesia cases include situs inversus).[citation needed]
Treatment
There are no standardized effective treatment strategies for the condition. Current therapies for PCD are extrapolated from Cystic Fibrosis and patients with non-CF bronchiectasis and lack validation for PCD-specific use.[17] Severe fatal respiratory failure can develop; long-term treatment with macrolides such as clarithromycin, erythromycin and azithromycin has been empirically applied for the treatment of primary ciliary dyskinesia in Japan, though controversial due to the effects of the medications.[18]
Prognosis
There is no reliable estimate of life expectancy for people with PCD.[19] The largest multi-center study of lung function in people with PCD across many European countries found strong evidence refuting a common assumption that it is a mild disease. This study found that lung function of people with PCD is comparable to those with cystic fibrosis in childhood but is better in young adulthood.[20] Both diseases, however, are progressive and lung function declines with age relative to peer groups. Survey data indicate that respiratory symptoms increase progressively and continuously beginning in the mid-20s relative to the population norm.[21]
Research
Research to further the understanding of cilia, with the future aims of functional restoration of motile cilia is advancing. However, charitable funding for medial research, particularly for rare disease is vital and in the UK contributes to more than 50% of grants. The UK registered charity PCD Research supports research into PCD worldwide, with the ultimate aim of funding potentially curative research.[22] Future promising avenues for functional replacement of cilia involve antisense, gene editing via CRISPR-Cas9 and mRNA therapies. At present there have only been a handful of interventional trials in PCD.[23]
History
The classic symptom combination associated with PCD was first described in 1904 by A. K. Siewert,[24] while Manes Kartagener published his first report on the subject in 1933.[25] The disorder is often now referred to as Siewert's syndrome or Siewert-Kartagener syndrome.[26]
References
- ^ a b "DNAI1 - Dynein axonemal intermediate chain 1 - Homo sapiens (Human) - DNAI1 gene & protein". www.uniprot.org. Retrieved 7 May 2022.
- ^ "PCDresearch.org". PCDresearch.org. Retrieved 2022-10-24.
- ^ Coren, M. E; Meeks, M; Morrison, I; Buchdahl, R. M; Bush, A (2002). "Primary ciliary dyskinesia: Age at diagnosis and symptom history". Acta Paediatrica. 91 (6): 667–9. doi:10.1080/080352502760069089. PMID 12162599.
- ^ "PCD Family Support Group : FAQs". Archived from the original on 2016-10-08. Retrieved 2012-03-04.[full citation needed]
- ^ McComb, Peter; Langley, Lynn; Villalon, Manuel; Verdugo, Pedro (September 1986). "The oviductal cilia and Kartagener's syndrome". Fertility and Sterility. 46 (3): 412–416. doi:10.1016/S0015-0282(16)49578-6. PMID 3488922.
- ^ Ceccaldi, P.-F.; Carré-Pigeon, F.; Youinou, Y.; Delépine, B.; Bryckaert, P.-E.; Harika, G.; Quéreux, C.; Gaillard, D. (May 2004). "Syndrome de Kartagener et stérilité : observation, diagnostic et prise en charge" [Kartagener's syndrome and infertility: observation, diagnosis and treatment]. Journal de Gynécologie Obstétrique et Biologie de la Reproduction (in French). 33 (3): 192–194. doi:10.1016/s0368-2315(04)96439-3. PMID 15170433.
- ^ a b Chodhari, R; Mitchison, H.M; Meeks, M (2004). "Cilia, primary ciliary dyskinesia and molecular genetics". Paediatric Respiratory Reviews. 5 (1): 69–76. doi:10.1016/j.prrv.2003.09.005. PMID 15222957.
- ^ GeneReviews. "Primary Ciliary Dyskinesia". Retrieved 2007-11-16.
- ^ a b Zariwala, Maimoona A; Knowles, Michael R; Omran, Heymut (2007). "Genetic Defects in Ciliary Structure and Function". Annual Review of Physiology. 69: 423–50. doi:10.1146/annurev.physiol.69.040705.141301. PMID 17059358.
- ^ Zariwala MA, Knowles MR, Leigh MW (2015) Primary Ciliary Dyskinesia. In: Adam MP, Ardinger HH, Pagon RA, Wallace SE, Bean LJH, Stephens K, Amemiya A, editors. GeneReviews. Seattle (WA): University of Washington, Seattle; 1993–2018
- ^ Bustamante-Marin, Ximena M.; Yin, Wei-Ning; Sears, Patrick R.; Werner, Michael E.; Brotslaw, Eva J.; Mitchell, Brian J.; Jania, Corey M.; Zeman, Kirby L.; Rogers, Troy D.; Herring, Laura E.; Refabért, Luc; Thomas, Lucie; Amselem, Serge; Escudier, Estelle; Legendre, Marie; Grubb, Barbara R.; Knowles, Michael R.; Zariwala, Maimoona A.; Ostrowski, Lawrence E. (2019). "Lack of GAS2L2 Causes PCD by Impairing Cilia Orientation and Mucociliary Clearance". The American Journal of Human Genetics. 104 (2): 229–245. doi:10.1016/j.ajhg.2018.12.009. PMC 6372263. PMID 30665704.
- ^ Cartwright, J. H. E; Piro, O; Tuval, I (2004). "Fluid-dynamical basis of the embryonic development of left-right asymmetry in vertebrates". Proceedings of the National Academy of Sciences. 101 (19): 7234–9. Bibcode:2004PNAS..101.7234C. doi:10.1073/pnas.0402001101. PMC 409902. PMID 15118088.
- ^ a b Nonaka, Shigenori; Yoshiba, Satoko; Watanabe, Daisuke; Ikeuchi, Shingo; Goto, Tomonobu; Marshall, Wallace F; Hamada, Hiroshi (2005). "De Novo Formation of Left–Right Asymmetry by Posterior Tilt of Nodal Cilia". PLOS Biology. 3 (8): e268. doi:10.1371/journal.pbio.0030268. PMC 1180513. PMID 16035921.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Kennedy, M. P; Omran, H; Leigh, M. W; Dell, S; Morgan, L; Molina, P. L; Robinson, B. V; Minnix, S. L; Olbrich, H; Severin, T; Ahrens, P; Lange, L; Morillas, H. N; Noone, P. G; Zariwala, M. A; Knowles, M. R (2007). "Congenital Heart Disease and Other Heterotaxic Defects in a Large Cohort of Patients with Primary Ciliary Dyskinesia". Circulation. 115 (22): 2814–21. doi:10.1161/CIRCULATIONAHA.106.649038. PMID 17515466.
- ^ Badano, Jose L; Mitsuma, Norimasa; Beales, Phil L; Katsanis, Nicholas (2006). "The Ciliopathies: An Emerging Class of Human Genetic Disorders". Annual Review of Genomics and Human Genetics. 7: 125–48. doi:10.1146/annurev.genom.7.080505.115610. PMID 16722803.
- ^ Shoemark, Amelia; Moya, Eduardo; Hirst, Robert A; Patel, Mitali P; Robson, Evelyn A; Hayward, Jane; Scully, Juliet; Fassad, Mahmoud R; Lamb, William; Schmidts, Miriam; Dixon, Mellisa; Patel-King, Ramila S; Rogers, Andrew V; Rutman, Andrew; Jackson, Claire L; Goggin, Patricia; Rubbo, Bruna; Ollosson, Sarah; Carr, Siobhán; Walker, Woolf; Adler, Beryl; Loebinger, Michael R; Wilson, Robert; Bush, Andrew; Williams, Hywel; Boustred, Christopher; Jenkins, Lucy; Sheridan, Eamonn; Chung, Eddie M K; et al. (2018). "High prevalence ofCCDC103p.His154Pro mutation causing primary ciliary dyskinesia disrupts protein oligomerisation and is associated with normal diagnostic investigations". Thorax. 73 (2): 157–166. doi:10.1136/thoraxjnl-2017-209999. PMC 5771957. PMID 28790179.
- ^ Knowles, Michael; Daniels, Leigh Anne; Davis, Stephanie; Zariwala, Maimoona; Leigh, Margaret (June 24, 2013). "Primary Ciliary Dyskinesia. Recent Advances in Diagnostics, Genetics, and Characterization of Clinical Disease". American Journal of Respiratory and Critical Care Medicine. 188 (8): 913–22. doi:10.1164/rccm.201301-0059CI. PMC 3826280. PMID 23796196.
- ^ Kido, Takashi; Yatera, Kazuhiro; Yamasaki, Kei; Nagata, Shuya; Choujin, Yasuo; Yamaga, Chiyo; Hara, Kanako; Ishimoto, Hiroshi; Hisaoka, Masanori; Mukae, Hiroshi (2012). "Two Cases of Primary Ciliary Dyskinesia with Different Responses to Macrolide Treatment". Internal Medicine. 51 (9): 1093–8. doi:10.2169/internalmedicine.51.6617. PMID 22576394.
- ^ "FREQUENTLY ASKED QUESTIONS Everything you need to know about primary ciliary dyskinesia (PCD)!". PCD Foundation. Retrieved 16 September 2018.
- ^ Halbeisen, Florian; Goutaki, Myrofora; Spycher, Ben; Amirav, Israel; Laura, Behan; Boon, Mieke; Hogg, Claire; Casa, Carmen; Crowl, Suzanne; Haarman, Eric; Bulent, Karadag (August 2018). "Lung function in patients with primary ciliary dyskinesia: an iPCD Cohort study". The European Respiratory Journal. 52 (2): 1801040. doi:10.1183/13993003.01040-2018. PMID 30049738.
- ^ McManus, I Christopher; Mitchison, Hannah M.; Chung, Eddie MK; Stubbings, Georgina F.; Martin, Naomi (2003). "Primary ciliary dyskinesia (Siewert's / Kartagener's Syndrome): Respiratory symptoms and psycho-social impact". BMC Pulmonary Medicine. 3: 4. doi:10.1186/1471-2466-3-4. PMC 317322. PMID 14641928.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ "PCDresearch.org". PCDresearch.org. Retrieved 2022-10-24.
- ^ "Search of: primary ciliary dyskinesia - List Results - ClinicalTrials.gov". clinicaltrials.gov. Retrieved 2022-10-24.
- ^ Siewert AK (8 February 1904). "Über einen Fall von Bronchiectasie bei einem Patienten mit situs inversus viscerum" [About a case of bronchiectasis in a patient avec situs inversus viscerum]. Berliner Klinische Wochenschrift (in German). 41 (6): 139–141.
- ^ Kartagener, M. (September 1933). "Zur Pathogenese der Bronchiektasien" [On the pathogenesis of bronchiectasis]. Beiträge zur Klinik der Tuberkulose und spezifischen Tuberkulose-Forschung (in German). 83 (4): 489–501. doi:10.1007/BF02141468. S2CID 7708592.
- ^ McManus, Chris (February 2004). "Eponymous but anonymous: who was Dr Siewert?". The Lancet. 363 (9409): 662. doi:10.1016/S0140-6736(04)15611-0. PMID 14987904. S2CID 25967658.
