Mearnsetin
Appearance
| |
| Names | |
|---|---|
| IUPAC name
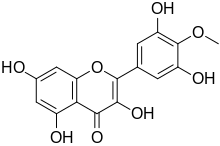
3,3′,5,5′,7-Pentahydroxy-4′-methoxyflavone
| |
| Systematic IUPAC name
2-(3,5-Dihydroxy-4-methoxyphenyl)-3,5,7-trihydroxy-4H-1-benzopyran-4-one | |
| Other names
4'-Methylmyricetin
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C16H12O8 | |
| Molar mass | 332.264 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Mearnsetin is an O-methylated flavonol. It can be found in Eucalyptus globulus and in Elaeocarpus lanceofolius.[1] The compound has antioxidative properties.[2]
Mearnsetin 3,7-dirhamnoside can be found in the fern Asplenium antiquum.[3]
References
[edit]- ^ Ray, A.B.; Dutta, S.C.; Dasgupta, S. (1976). "Flavonoids of Elaeocarpus lanceofolius". Phytochemistry. 15 (11): 1797–1798. doi:10.1016/S0031-9422(00)97498-3.
- ^ Sadasivam, K.; Kumaresan, R. (2011). "Antioxidant behavior of mearnsetin and myricetin flavonoid compounds--a DFT study". Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy. 79 (1): 282–93. Bibcode:2011AcSpA..79..282S. doi:10.1016/j.saa.2011.02.042. PMID 21420896.
- ^ Mizuno, Mizuo; Kyotani, Yosuke; Iinuma, Munekazu; Tanaka, Toshiyuki; Kojima, Hiroyuki; Iwatsuki, Kunio (1991). "Mearnsetin 3,7-dirhamnoside from Asplenium antiquum". Phytochemistry. 30 (8): 2817–2818. doi:10.1016/0031-9422(91)85158-V.
