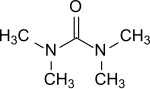
Tetramethylurea
| |
| Names | |
|---|---|
| Preferred IUPAC name
Tetramethylurea | |
| Other names
1,1,3,3-Tetramethylurea
*TMU | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.010.159 |
| EC Number |
|
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C5H12N2O | |
| Molar mass | 116.164 g·mol−1 |
| Appearance | Colorless liquid |
| Density | 0.968 g/mL |
| Melting point | −1.2 °C (29.8 °F; 271.9 K) |
| Boiling point | 176.5 °C (349.7 °F; 449.6 K) |
| Hazards | |
| GHS labelling: | |
 
| |
| Danger | |
| H302, H360, H361 | |
| P201, P202, P264, P270, P281, P301+P312, P308+P313, P330, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Tetramethylurea is the organic compound with the formula (Me2N)2CO. It is a substituted urea. This colorless liquid is used as an aprotic-polar solvent, especially for aromatic compounds and is used e. g. for Grignard reagents.[1]
Production
The synthesis and properties of tetramethylurea were comprehensively described.[1]
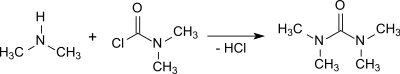
The reaction of dimethylamine with phosgene in the presence of e. g. 50 % sodium hydroxide solution and subsequent extraction with 1,2-dichloroethane yields tetramethylurea in 95% yield.[2]

The reactions with dimethylcarbamoyl chloride or phosgene are highly exothermic and the removal of the resulting dimethylamine hydrochloride requires some effort.[1]
The reaction of diphenylcarbonate with dimethylamine in an autoclave is also effective.

Tetramethylurea is formed in the reaction of dimethylcarbamoyl chloride with anhydrous sodium carbonate in a yield of 96.5%.[3]
Dimethylcarbamoyl chloride also reacts with excess dimethylamine forming tetramethylurea. Even though the product is contaminated and smelly it may be purified by addition of calcium oxide and subsequent fractional distillation.[4]
Tetramethylurea is also formed during the oxidation of tetrakis(dimethylamino)ethylene (TDAE), a very electron-rich alkene[5] and a strong reducing agent, available from tris(dimethylamino)methane by pyrolysis[6] or from chlorotrifluoroethene and dimethylamine.[7]

Tetrakis(dimethylamino)ethylene (TDAE) reacts with oxygen in a (2+2) cycloaddition reaction to a 1,2-dioxetane which decomposes to electronically excited tetramethylurea. This returns to the ground state while emitting green light with an emission maximum at 515 nm.[8][9]

Properties
Tetramethylurea is a clear, colorless liquid with mild aromatic odor that is miscible with water and many organic solvents.[10] Unusual for an urea is the liquid state of tetramethylurea in a range of > 170 °C.
Applications
Tetramethylurea is miscible with a variety of organic compounds, including acids such as acetic acid or bases such as pyridine and an excellent solvent for organic substances such as ε-caprolactam or benzoic acid and dissolves even some inorganic salts such as silver nitrate or sodium iodide.[11][12] Due to its distinct solvent properties tetramethylurea is often used as a replacement for the carcinogenic hexamethylphosphoramide (HMPT).[13]
Tetramethylurea is suitable as a reaction medium for the polymerization of aromatic diacid chlorides (such as isophthalic acid) and aromatic diamines (such as 1,3-diaminobenzene (m-phenylenediamine)) to aramids such as poly (m-phenylene isophthalamide) (Nomex®)[14][15]
The polymerization of 4-amino benzoic acid chloride hydrochloride in tetramethylurea provides isotropic viscous solutions of poly(p-benzamide) (PPB), which can be directly spun into fibers.[16]

In a tetramethylurea-LiCl mixture stable isotropic solutions can be obtained up to a PPB polymer concentration of 14%.[17]
Tetramethylurea also dissolves cellulose ester and swells other polymers such as polycarbonates, polyvinyl chloride or aliphatic polyamides, usually at elevated temperature.[1]
Strong and hindered non-nucleophilic guanidine bases are accessible from tetramethylurea in a simple manner,[18][19] which are in contrast to the fused amidine bases DBN or DBU not alkylated.

A modification of the Koenigs-Knorr reaction for building glycosides from 2,3,4,6-tetra-O-acetyl-α-D-glucopyranosyl bromide (acetobromoglucose) originates from S. Hanessian who used the silver salt silver trifluoromethanesulfonate (TfOAg) and as a proton acceptor tetramethylurea.[20] This process variant is characterized by a simplified process control, high anomeric purity and high yields of the products. If the reaction is carried out with acetobromoglucose and silver triflate/tetramethylurea at room temperature, then tetramethylurea reacts not only as a base, but also with the glycosyl to form a good isolable uroniumtriflates in 56% yield.[21]

Safety
The acute toxicity of tetramethylurea is moderate. However, it is embryotoxic and teratogenic towards several animal species.[22]
References
- ^ a b c d A. Lüttringhaus; H.-W. Dirksen (1963), "Tetramethylharnstoff als Lösungsmittel und Reaktionspartner", Angew. Chem. (in German), vol. 75, no. 22, pp. 1059–1068, Bibcode:1963AngCh..75.1059L, doi:10.1002/ange.19630752204
- ^ US 3681457, H. Babad, "Method of making tetramethylurea", published 1972-8-1, assigned to The Ott Chemical Co.
- ^ J.K. Lawson Jr.; J.A.T. Croom (1963), "Dimethylamides from alkali carboxylates and dimethylcarbamoyl chloride", J. Org. Chem. (in German), vol. 28, no. 1, pp. 232–235, doi:10.1021/jo01036a513
- ^ US 3597478, M.L. Weakly, "Preparation of tetramethylurea", published 1971-8-3, assigned to Nipak, Inc.
- ^ H. Bock; H. Borrmann; Z. Havlas; H. Oberhammer; K. Ruppert; A. Simon (1991), "Tetrakis(dimethylamino)ethen: Ein extrem elektronenreiches Molekül mit ungewöhnlicher Struktur sowohl im Festkörper als auch in der Gasphase", Angew. Chem. (in German), vol. 103, no. 12, pp. 1733–1735, Bibcode:1991AngCh.103.1733B, doi:10.1002/ange.19911031246
- ^ H. Weingarten; W.A. White (1966), "Synthesis of Tetrakis(dimethylamino)ethylene", J. Org. Chem. (in German), vol. 31, no. 10, pp. 3427–3428, doi:10.1021/jo01348a520
- ^ US 3293299, H. Boden, "Process for making tetrakis(dimethylamino)ethylene", published 1966-12-20, assigned to E.I. du Pont de Nemours and Co.
- ^ H.E. Winberg; J.R. Downing; D.D. Coffman (1965), "The chemiluminescence of tetrakis(dimethylamino)ethylene", J. Am. Chem. Soc. (in German), vol. 87, no. 9, pp. 2054–2055, doi:10.1021/ja01087a039
- ^ "Chemilumineszenz von TDAE" (in German). illumina-chemie.de. 2014-08-08. Retrieved 2016-08-22.
- ^ R.M. Giuliano (2004). "Tetramethylurea". Encyclopedia of Reagents for Organic Synthesis. doi:10.1002/047084289X.rn00399. ISBN 978-0-471-93623-7.
- ^ B.J. Barker; J.A. Caruso (1976), The Chemistry of Nonaqueous Solvents, IV. Solution Phenomena and Aprotic Solvents (in German), New York: Academic Press, pp. 110–127, ISBN 978-0-12-433804-3
- ^ B.J. Barker; J. Rosenfarb; J.A. Caruso (1979), "Harnstoffe als Lösungsmittel in der chemischen Forschung", Angew. Chem. (in German), vol. 91, no. 7, pp. 560–564, Bibcode:1979AngCh..91..560B, doi:10.1002/ange.19790910707
- ^ A.J. Chalk (1970), "The use of sodium hydride as a reducing agent in nitrogen-containing solvents I. The reduction of chlorosilanes in Hexaalkylphosphoric triamides and tetraalkylureas", J. Organomet. Chem. (in German), vol. 21, no. 1, pp. 95–101, doi:10.1016/S0022-328X(00)90598-9
- ^ G. Odian (2004), Principles of Polymerization, 4th Edition (in German), Hoboken, NJ: Wiley-Interscience, p. 100, ISBN 978-0-471-27400-1
- ^ H.G. Rodgers; R.A. Gaudiana; W.C. Hollinsed; P.S. Kalyanaraman; J.S. Manello; C. McGovern; R.A. Minns; R. Sahatjian (1985), "Highly amorphous, birefringent, para-linked aromatic polyamides", Macromolecules (in German), vol. 18, no. 6, pp. 1058–1068, Bibcode:1985MaMol..18.1058R, doi:10.1021/ma00148a003
- ^ J. Preston (1978), A. Blumstein (ed.), Synthesis and Properties of Rodlike Condensation Polymers, in Liquid Crystalline Order in Polymers (in German), New York: Academic Press, pp. 141–166, ISBN 978-0-12-108650-3
- ^ S.L. Kwolek; P.W. Morgan; J.R. Schaefgen; L.W. Gulrich (1977), "Synthesis, Anisotropic Solutions, and Fibers of Poly(1,4-benzamide)", Macromolecules (in German), vol. 10, no. 6, pp. 1390–1396, Bibcode:1977MaMol..10.1390K, doi:10.1021/ma60060a041
- ^ D.H.R. Barton; M. Chen; J.C. Jászbérenyi; D.K. Taylor (1997). "Preparation and Reactions of 2-tert-butyl-1,1,3,3-tetramethylguanidine: 2,2,6-trimethylcyclohexen-1-yl iodide". Organic Syntheses. 74: 101. doi:10.15227/orgsyn.074.0101.
- ^ D.H.R. Barton; J.D. Elliott; S.D. Géro (1981), "The synthesis and properties of a series of strong but hindered organic bases", J. Chem. Soc., Chem. Commun. (in German), no. 21, pp. 1136–1137, doi:10.1039/C39810001136
- ^ S. Hanessian; J. Banoub (1977), "Chemistry of the glycosidic linkage. An efficient synthesis of 1,2-trans-disaccharides", Carbohydr. Res. (in German), vol. 53, pp. C13–C16, doi:10.1016/S0008-6215(00)85468-3
- ^ K. Bock; J. Fernández-Bolanos Guzmán; S. Refn (1992), "Synthesis and properties of 1,1,3,3-tetramethyl-2-(2,3,4,6-tetra-O-acetyl-α-D-glucopyranosyl)uronium triflate", Carbohydr. Res. (in German), vol. 232, no. 2, pp. 353–357, doi:10.1016/0008-6215(92)80067-B
- ^ The MAK Collection for Occupational Health and Safety (2012), "Tetramethylharnstoff [MAK Value Documentation in German language, 1979]", Tetramethylharnstoff [MAK Value Documentation in German language, 1979], Documentations and Methods (in German), Weinheim: Wiley-VCH, pp. 1–6, doi:10.1002/3527600418.mb63222d0007, ISBN 978-3-527-60041-0
