Free-radical theory of aging
This article needs more reliable medical references for verification or relies too heavily on primary sources. (May 2015) |  |
The free radical theory of aging (FRTA) states that organisms age because cells accumulate free radical damage over time.[1] A free radical is any atom or molecule that has a single unpaired electron in an outer shell.[2] While a few free radicals such as melanin are not chemically reactive, most biologically relevant free radicals are highly reactive.[3] For most biological structures, free radical damage is closely associated with oxidative damage. Antioxidants are reducing agents, and limit oxidative damage to biological structures by passivating them from free radicals.[4]
Strictly speaking, the free radical theory is only concerned with free radicals such as superoxide ( O2− ), but it has since been expanded to encompass oxidative damage from other reactive oxygen species such as hydrogen peroxide (H2O2), or peroxynitrite (OONO−).[4]
Denham Harman first proposed the free radical theory of aging in the 1950s,[5] and in the 1970s extended the idea to implicate mitochondrial production of reactive oxygen species.[6]
In some model organisms, such as yeast and Drosophila, there is evidence that reducing oxidative damage can extend lifespan.[7] However, in mice, only 1 of the 18 genetic alterations (SOD-1 deletion) that block antioxidant defences, shortened lifespan.[8] Similarly, in roundworms (Caenorhabditis elegans), blocking the production of the naturally occurring antioxidant superoxide dismutase has recently been shown to increase lifespan.[9] Whether reducing oxidative damage below normal levels is sufficient to extend lifespan remains an open and controversial question.
Background
The free radical theory of aging was conceived by Denham Harman in the 1950s, when prevailing scientific opinion held that free radicals were too unstable to exist in biological systems.[10] This was also before anyone invoked free radicals as a cause of degenerative diseases.[11] Two sources inspired Harman: 1) the rate of living theory, which holds that lifespan is an inverse function of metabolic rate which in turn is proportional to oxygen consumption, and 2) Rebbeca Gershman's observation that hyperbaric oxygen toxicity and radiation toxicity could be explained by the same underlying phenomenon: oxygen free radicals.[10][12] Noting that radiation causes "mutation, cancer and aging", Harman argued that oxygen free radicals produced during normal respiration would cause cumulative damage which would eventually lead to organismal loss of functionality, and ultimately death.[10][12]
In later years, the free radical theory was expanded to include not only aging per se, but also age-related diseases.[11] Free radical damage within cells has been linked to a range of disorders including cancer, arthritis, atherosclerosis, Alzheimer's disease, and diabetes.[13] There has been some evidence to suggest that free radicals and some reactive nitrogen species trigger and increase cell death mechanisms within the body such as apoptosis and in extreme cases necrosis.[14]
In 1972, Harman modified his original theory.[11] In its current form, this theory proposes that reactive oxygen species that are produced in the mitochondria, causes damage to certain macromolecules including lipids, proteins and most importantly mitochondrial DNA.[15] This damage then causes mutations which lead to an increase of ROS production and greatly enhance the accumulation of free radicals within cells.[15] This mitochondrial theory has been more widely accepted that it could play a major role in contributing to the aging process.[16]
Since Harman first proposed the free radical theory of aging, there have been continual modifications and extensions to his original theory.[16]
Processes
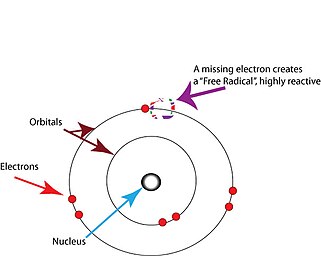
Free radicals are atoms or molecules containing unpaired electrons.[2] Electrons normally exist in pairs in specific orbitals in atoms or molecules.[17] Free radicals, which contain only a single electron in any orbital, are usually unstable toward losing or picking up an extra electron, so that all electrons in the atom or molecule will be paired.[17]
Note that the unpaired electron does not imply charge - free radicals can be positively charged, negatively charged, or neutral.
Damage occurs when the free radical encounters another molecule and seeks to find another electron to pair its unpaired electron. The free radical often pulls an electron off a neighboring molecule, causing the affected molecule to become a free radical itself. The new free radical can then pull an electron off the next molecule, and a chemical chain reaction of radical production occurs.[18] The free radicals produced in such reactions often terminate by removing an electron from a molecule which becomes changed or cannot function without it, especially in biology. Such an event causes damage to the molecule, and thus to the cell that contains it (since the molecule often becomes dysfunctional).
The chain reaction caused by free radicals can lead to cross-linking of atomic structures. In cases where the free radical-induced chain reaction involves base pair molecules in a strand of DNA, the DNA can become cross-linked.[19]
DNA cross-linking can in turn lead to various effects of aging, especially cancer.[20] Other cross-linking can occur between fat and protein molecules, which leads to wrinkles.[21] Free radicals can oxidize LDL, and this is a key event in the formation of plaque in arteries, leading to heart disease and stroke.[22] These are examples of how the free-radical theory of aging has been used to neatly "explain" the origin of many chronic diseases.[23]
Free radicals that are thought to be involved in the process of aging include superoxide and nitric oxide.[24] Specifically, an increase in superoxide affects aging whereas a decrease in nitric oxide formation, or its bioavailability, does the same.[24]
Antioxidants are helpful in reducing and preventing damage from free radical reactions because of their ability to donate electrons which neutralize the radical without forming another. Ascorbic acid, for example, can lose an electron to a free radical and remain stable itself by passing its unstable electron around the antioxidant molecule.[25]
This has led to the hypothesis that large amounts of antioxidants,[26] with their ability to decrease the numbers of free radicals, might lessen the radical damage causing chronic diseases, and even radical damage responsible for aging.
Evidence
Numerous studies have demonstrated a role for free radicals in the aging process and thus tentatively support the free radical theory of aging. Studies have shown a significant increase in superoxide radical (SOR) formation and lipid peroxidation in aging rats.[27] Chung et al. suggest ROS production increases with age and indicated the conversion of XDH to XOD may be an important contributing factor.[28] This was supported by a study that showed superoxide production by xanthine oxidase and NO synthase in mesenteric arteries was higher in older rats than young ones.[29]
Hamilton et al. examined the similarities in impaired endothelial function in hypertension and aging in humans and found a significant overproduction of superoxide in both.[30] This finding is supported by a 2007 study which found that endothelial oxidative stress develops with aging in healthy men and is related to reductions in endothelium-dependent dilation.[31] Furthermore, a study using cultured smooth muscle cells displayed increased reactive oxygen species (ROS) in cells derived from older mice.[32] These findings were supported by a second study using Leydig cells isolated from the testes of young and old rats.[33]
The Choksi et al. experiment with Ames dwarf (DW) mice suggests the lower levels of endogenous ROS production in DW mice may be a factor in their resistance to oxidative stress and long life.[34] Lener et al. suggest Nox4 activity increases oxidative damage in human umbilical vein endothelial cells via superoxide overproduction.[35] Furthermore, Rodriguez-Manas et al. found endothelial dysfunction in human vessels is due to the collective effect of vascular inflammation and oxidative stress.[36]
Sasaki et al. reported superoxide-dependent chemiluminescence was inversely proportionate to maximum lifespan in mice, Wistar rats, and pigeons.[37] They suggest ROS signalling may be a determinant in the aging process.[37] In humans, Mendoza-Nunez et al. propose an age of 60 years or older may be linked with increased oxidative stress.[38] Miyazawa found mitochondrial superoxide anion production can lead to organ atrophy and dysfunction via mitochondrial- mediated apoptosis.[39] In addition, they suggest mitochondrial superoxide anion plays an essential part in aging.[40] Lund et al. demonstrated the role of endogenous extracellular superoxide dismutase in protecting against endothelial dysfunction during the aging process using mice.[41]
Modifications of the free radical theory of aging
One of the main criticisms of the free radical theory of aging is directed at the suggestion that free radicals are responsible for the damage of biomolecules, thus being a major reason for cellular senescence and organismal aging.[42]: 81 Several modifications have been proposed to integrate current research into the overall theory.
Mitochondrial theory of aging

Mitochondrial theory of aging was first proposed in 1978,[43][44] and shortly thereafter the Mitochondrial free radical theory of aging was introduced in 1980.[45] The theory implicates the mitochondria as the chief target of radical damage, since there is a known chemical mechanism by which mitochondria can produce Reactive oxygen species (ROS), mitochondrial components such as mtDNA are not as well protected as nuclear DNA, and by studies comparing damage to nuclear and mtDNA that demonstrate higher levels of radical damage on the mitochondrial molecules.[46] Electrons may escape from metabolic processes in the mitochondria like the Electron transport chain, and these electrons may in turn react with water to form ROS such as the superoxide radical, or via an indirect route the hydroxyl radical. These radicals then damage the mitochondria's DNA and proteins, and these damage components in turn are more liable to produce ROS byproducts. Thus a positive feedback loop of oxidative stress is established that, over time, can lead to the deterioration of cells and later organs and the entire body.[42]
This theory has been widely debated[47] and it is still unclear how ROS induced mtDNA mutations develop.[42] Conte et al. suggest iron-substituted zinc fingers may generate free radicals due the zinc finger proximity to DNA and thus lead to DNA damage.[48]
Afanas'ev suggests the superoxide dismutation activity of CuZnSOD demonstrates an important link between life span and free radicals.[49] The link between CuZnSOD and life span was demonstrated by Perez et al. who indicated mice life span was affected by the deletion of the Sod1 gene which encodes CuZnSOD.[50]
Contrary to the usually observed association between mitochondrial ROS (mtROS) and a decline in longevity, Yee et al. recently observed increased longevity mediated by mtROS signaling in an apoptosis pathway. This serves to support the possibility that observed correlations between ROS damage and aging are not necessarily indicative of the causal involvement of ROS in the aging process but are more likely due to their modulating signal transduction pathways that are part of cellular responses to the aging process.[51]
Epigenetic oxidative redox shift (EORS) theory of aging
Brewer proposed a theory which integrates the free radical theory of aging with the insulin signalling effects in aging.[52] Brewer's theory suggests "sedentary behaviour associated with age triggers an oxidized redox shift and impaired mitochondrial function".[52] This mitochondrial impairment leads to more sedentary behaviour and accelerated aging.[52]
Metabolic stability theory of aging
The metabolic stability theory of aging suggests it is the cells ability to maintain stable concentration of ROS which is the primary determinant of lifespan.[53] This theory criticizes the free radical theory because it ignores that ROS are specific signalling molecules which are necessary for maintaining normal cell functions.[53]
Mitohormesis
Oxidative stress may promote life expectancy of Caenorhabditis elegans by inducing a secondary response to initially increased levels of reactive oxygen species.[54] In mammals, the question of the net effect of reactive oxygen species on aging is even less clear.[55][56][57] Recent epidemiological findings support the process of mitohormesis in humans, and even suggest that the intake of exogenous antioxidants may increase disease prevalence in humans (according to the theory, because they prevent the stimulation of the organism's natural response to the oxidant compounds which not only neutralizes them but provides other benefits as well).[58]
Effects of calorie restriction
Studies have demonstrated that calorie restriction displays positive effects on the lifespan of organisms even though it is accompanied by increases in oxidative stress.[49] Many studies suggest this may be due to anti-oxidative action,[49] oxidative stress suppression,[59] or oxidative stress resistance[60] which occurs in calorie restriction. Fontana et al. suggest calorie restriction influenced numerous signal pathways through the reduction of insulin-like growth factor I (IGF-1).[61] Additionally they suggest antioxidant SOD and catalase are involved in the inhibition of this nutrient signalling pathway.[61]
The increase in life expectancy observed during some calorie restriction studies which can occur with lack of decreases or even increases in O2 consumption is often inferred as opposing the mitochondrial free radical theory of aging.[49][62] However, Barja showed significant decreases in mitochondrial oxygen radical production (per unit of O2 consumed) occur during dietary restriction, aerobic exercise, chronic exercise, and hyperthyroidism.[62] Additionally, mitochondrial oxygen radical generation is lower in long-lived birds than in short-lived mammals of comparable body size and metabolic rate. Thus, mitochondrial ROS production must be regulated independently of O2 consumption in a variety of species, tissues and physiologic states.[62]
Challenges to the free radical theory of aging
Naked Mole-rat
The naked mole-rat is a long-lived (32 years) rodent. As reviewed by Lewis et al.,[63] (2013), levels of reactive oxygen species (ROS) production in the naked mole rat are similar to that of another rodent, the relatively short-lived mouse (4 years). They concluded that it is not oxidative stress that modulates health-span and longevity in these rodents, but rather other cytoprotective mechanisms that allow animals to deal with high levels of oxidative damage and stress.[63] In the naked mole-rat, a likely important cytoprotective mechanism that could provide longevity assurance is elevated expression of DNA repair genes involved in several key DNA repair pathways.[64] (See DNA damage theory of aging.) Compared with the mouse, the naked mole rat had significantly higher expression levels of genes essential for the DNA repair pathways of DNA mismatch repair, non-homologous end joining and base excision repair.[64]
Birds
Among birds, parrots live about 5-times longer than quail. Reactive oxygen species (ROS) production in heart, skeletal muscle, liver and intact erythrocytes was found to be similar in parrots and quail and showed no correspondence with longevity difference.[65] These findings were concluded to cast doubt on the robustness of the oxidative stress theory of aging.[65]
See also
- American Aging Association
- Life extension
- List of life extension-related topics
- Senescence
- Calorie restriction
- Denham Harman
- Mitochondrial theory of ageing
References
- ^ Hekimi S, Lapointe J, Wen Y. Taking a "good" look at free radicals in the aging process. Trends In Cell Biology. 2011;21(10) 569-76.
- ^ a b Erbas M, Sekerci H. IMPORTANCE OF FREE RADICALS AND OCCURRING DURING FOOD PROCESSING. SERBEST RADÏKALLERÏN ONEMÏ VE GIDA ÏSLEME SIRASINDA OLUSUMU. 2011;36(6) 349-56.
- ^ Herrling T, Jung K, Fuchs J (2008). "The role of melanin as protector against free radicals in skin and its role as free radical indicator in hair". Spectrochimica Acta Part A: Molecular & Biomolecular Spectroscopy. 69 (5): 1429–35. Bibcode:2008AcSpA..69.1429H. doi:10.1016/j.saa.2007.09.030. PMID 17988942.
- ^ a b Halliwell B (2012). "Free radicals and antioxidants: updating a personal view". Nutrition Reviews. 70 (5): 257–65. doi:10.1111/j.1753-4887.2012.00476.x. PMID 22537212.
- ^ Harman, D (1956). "Aging: a theory based on free radical and radiation chemistry". Journal of Gerontology. 11 (3): 298–300. doi:10.1093/geronj/11.3.298. PMID 13332224.
- ^ Harman, D (1972). "A biologic clock: the mitochondria?". Journal of the American Geriatrics Society. 20 (4): 145–147. doi:10.1111/j.1532-5415.1972.tb00787.x. PMID 5016631.
- ^ Fontana, Luigi; Partridge, Linda; Longo, Valter D. (16 April 2010). "Extending Healthy Life Span—From Yeast to Humans". Science. 328 (5976): 321–326. Bibcode:2010Sci...328..321F. doi:10.1126/science.1172539. PMC 3607354. PMID 20395504.
- ^ Pérez VI, Bokov A, Remmen HV, Mele J, Ran Q, Ikeno Y, et al. (2009). "Is the oxidative stress theory of aging dead?". Biochimica et Biophysica Acta (BBA) - General Subjects. 1790 (10): 1005–14. doi:10.1016/j.bbagen.2009.06.003. PMC 2789432. PMID 19524016.
- ^ Van Rammsdonk, Jeremy M.; Hekimi, Siegfried (2009). Kim, Stuart K. (ed.). "Deletion of the Mitochondrial Superoxide Dismutase sod-2 Extends Lifespan in Caenorhabditis elegans". PLOS Genetics. 5 (2): e1000361. doi:10.1371/journal.pgen.1000361. PMC 2628729. PMID 19197346.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ a b c Harman D (Jul 1956). "Aging: a theory based on free radical and radiation chemistry". J Gerontol. 11 (3): 298–300. doi:10.1093/geronj/11.3.298. PMID 13332224.
- ^ a b c Harman D (2009). "Origin and evolution of the free radical theory of aging: a brief personal history, 1954–2009". Biogerontology. 10 (6): 773–81. doi:10.1007/s10522-009-9234-2. PMID 19466577.
- ^ a b Speakman JR, Selman C (2011). "The free-radical damage theory: Accumulating evidence against a simple link of oxidative stress to ageing and lifespan". BioEssays. 33 (4): 255–9. doi:10.1002/bies.201000132. PMID 21290398.
- ^ Clancy D, Birdsall J. Flies, worms and the Free Radical Theory of ageing. Ageing Research Reviews. (0).
- ^ Chatterjee S, Lardinois O, Bhattacharjee S, Tucker J, Corbett J, Deterding L, et al. (2011). "Oxidative stress induces protein and DNA radical formation in follicular dendritic cells of the germinal center and modulates its cell death patterns in late sepsis". Free Radical Biology and Medicine. 50 (8): 988–99. doi:10.1016/j.freeradbiomed.2010.12.037. PMC 3051032. PMID 21215311.
- ^ a b Jang YC, Remmen HV (2009). "The mitochondrial theory of aging: Insight from transgenic and knockout mouse models". Experimental Gerontology. 44 (4): 256–60. doi:10.1016/j.exger.2008.12.006. PMID 19171187.
- ^ a b Gruber J, Schaffer S, Halliwell B (2008). "The mitochondrial free radical theory of ageing--where do we stand?". Frontiers in Bioscience. 13 (13): 6554–79. doi:10.2741/3174. PMID 18508680.
- ^ a b Orchin M, Macomber RS, Pinhas A, Wilson RM, editors. The Vocabulary and Concepts of Organic Chemistry. 2 ed: John Wiley & Sons; 2005.
- ^ Cui Hang; Kong Yahui; Zhang Hong (2011). "Oxidative Stress, Mitochondrial Dysfunction, and Aging". Journal of Signal Transduction. 2012: 646354. doi:10.1155/2012/646354. PMC 3184498. PMID 21977319.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Crean C, Geacintov NE, Shafirovich V (2008). "Intrastrand G-U cross-links generated by the oxidation of guanine in 5′-d(GCU) and 5′-r(GCU)". Free Radical Biology and Medicine. 45 (8): 1125–34. doi:10.1016/j.freeradbiomed.2008.07.008. PMC 2577587. PMID 18692567.
- ^ Dizdaroglu M, Jaruga P. Mechanisms of free radical-induced damage to DNA. Free Radical Research. [Article]. 2012;46(4) 382-419.
- ^ Pageon H, Asselineau D. An in Vitro Approach to the Chronological Aging of Skin by Glycation of the Collagen: The Biological Effect of Glycation on the Reconstructed Skin Model" Annals of the New York Academy of Sciences 2005;1043(1) 529-32.
- ^ Bamm VV, Tsemakhovich VA, Shaklai N. Oxidation of low-density lipoprotein by hemoglobin–hemichrome. The International Journal of Biochemistry & Cell Biology. 2003;35(3) 349-58.
- ^ C. Richter, JW Park, BN Ames "Normal oxidative damage to mitochondrial and nuclear DNA is extensive" "PNAS", 1988.
- ^ a b Afanas'ev IB (2005). "Free radical mechanisms of aging processes under physiological conditions". Biogerontology. 6 (4): 283–90. doi:10.1007/s10522-005-2626-z. PMID 16333762.
- ^ Bagchi D. et al "Oxygen free radical scavenging abilities of vitamins C and E, and a grape seed proanthocyanidin extract in vitro" "Research Communications in Molecular Pathology and Pharmacology" 1997.
- ^ Biesalski H. Free radical theory of aging. Current Opinion in Clinical Nutrition and Metabolic Care. 2002 January 2002;5(1) 5 -10.
- ^ Sawada M, Carlson JC (1987). "Changes in superoxide radical and lipid peroxide formation in the brain, heart and liver during the lifetime of the rat". Mechanisms of Ageing and Development. 41 (1–2): 125–37. doi:10.1016/0047-6374(87)90057-1. PMID 2828774.
- ^ Chung HY, Song SH, Kim HJ, Ikeno Y, Yu BP (1999). "Modulation of renal xanthine oxidoreductase in aging: gene expression and reactive oxygen species generation". The Journal of Nutrition, Health & Aging. 3 (1): 19–23. PMID 10888479.
- ^ Jacobson A, Yan C, Gao Q, Rincon-Skinner T, Rivera A, Edwards J, et al. (2007). "Aging enhances pressure-induced arterial superoxide formation". American Journal of Physiology. Heart and Circulatory Physiology. 293 (3): H1344–H50. doi:10.1152/ajpheart.00413.2007. PMC 4536921. PMID 17557915.
- ^ Hamilton CA, Brosnan MJ, McIntyre M, Graham D, Dominiczak AF (2001). "Superoxide excess in hypertension and aging: a common cause of endothelial dysfunction". Hypertension. 37 (2 Pt 2): 529–34. doi:10.1161/01.hyp.37.2.529. PMID 11230330.
- ^ Donato AJ, Eskurza I, Silver AE, Levy AS, Pierce GL, Gates PE, et al. (2007). "Direct evidence of endothelial oxidative stress with aging in humans: relation to impaired endothelium-dependent dilation and upregulation of nuclear factor-kappaB". Circulation Research. 100 (11): 1659–66. doi:10.1161/01.res.0000269183.13937.e8. PMID 17478731.
- ^ Moon SK, Thompson LJ, Madamanchi N, Ballinger S, Papaconstantinou J, Horaist C, et al. (2001). "Aging, oxidative responses, and proliferative capacity in cultured mouse aortic smooth muscle cells". American Journal of Physiology. Heart and Circulatory Physiology. 280 (6): H779–H88. doi:10.1152/ajpheart.2001.280.6.h2779. PMID 11356636.
- ^ Chen H, Cangello D, Benson S, Folmer J, Zhu H, Trush MA, et al. (2001). "Age-related increase in mitochondrial superoxide generation in the testosterone-producing cells of Brown Norway rat testes: relationship to reduced steroidogenic function?". Experimental Gerontology. 36 (8): 1361–73. doi:10.1016/s0531-5565(01)00118-8. PMID 11602210.
- ^ Choksi KB, Roberts LJ, DeFord JH, Rabek JP, Papaconstantinou J (2007). "Lower levels of F2-isoprostanes in serum and livers of long-lived Ames dwarf mice". Biochemical and Biophysical Research Communications. 364 (4): 761–4. doi:10.1016/j.bbrc.2007.10.100. PMC 2238179. PMID 17964285.
- ^ Lener B, Kozieł R, Pircher H, Hütter E, Greussing R, Herndler-Brandstetter D, et al. (2009). "The NADPH oxidase Nox4 restricts the replicative lifespan of human endothelial cells". The Biochemical Journal. 423 (3): 363–74. doi:10.1042/bj20090666. PMC 2762686. PMID 19681754.
- ^ Rodríguez-Mañas L, El-Assar M, Vallejo S, López-Dóriga P, Solís J, Petidier R, et al. (2009). "Endothelial dysfunction in aged humans is related with oxidative stress and vascular inflammation". Aging Cell. 8 (3): 226–38. doi:10.1111/j.1474-9726.2009.00466.x. PMID 19245678.
- ^ a b Sasaki T, Unno K, Tahara S, Shimada A, Chiba Y, Hoshino M, et al. (2008). "Age-related increase of superoxide generation in the brains of mammals and birds". Aging Cell. 7 (4): 459–69. doi:10.1111/j.1474-9726.2008.00394.x. PMID 18419797.
- ^ Mendoza-Núñez VM, Ruiz-Ramos M, Sánchez-Rodríguez MA, Retana-Ugalde R, Muñoz-Sánchez JL. Aging-related oxidative stress in healthy humans. The Tohoku Journal of Experimental Medicine. 2007;213(3) 261-8.
- ^ Miyazawa M, Ishii T, Yasuda K, Noda S, Onouchi H, Hartman PS, et al. (2009). "The role of mitochondrial superoxide anion (O2(-)) on physiological aging in C57BL/6J mice". Journal of Radiation Research. 50 (1): 73–83. Bibcode:2009JRadR..50...73M. doi:10.1269/jrr.08097. PMID 19218782.
- ^ Miyazawa M, Ishii T, Yasuda K, Noda S, Onouchi H, Hartman PS, et al.
- ^ Lund DD, Chu Y, Miller JD, Heistad DD (2009). "Protective effect of extracellular superoxide dismutase on endothelial function during aging". American Journal of Physiology. Heart and Circulatory Physiology. 296 (6): H1920–H5. doi:10.1152/ajpheart.01342.2008. PMC 2716111. PMID 19376805.
- ^ a b c Afanas'ev I (2010). "Signaling and Damaging Functions of Free Radicals in Aging-Free Radical Theory, Hormesis, and TOR". Aging and Disease. 1 (2): 75–88. PMC 3295029. PMID 22396858.
- ^ Lobachev A.N.Role of mitochondrial processes in the development and aging of organism. Aging and cancer (PDF), Chemical abstracts. 1979 v. 91 N 25 91:208561v.Deposited Doc., VINITI 2172-78, 1978, p. 48
- ^ Lobachev A.N.Biogenesis of mitochondria during cell differentiation and aging (PDF), Deposited Doc. VINITI 19.09.85, №6756-В85, 1985, p. 28
- ^ Miquel J, Economos AC, Fleming J, et al.Mitochondrial role in cell aging, Exp Gerontol, 15, 1980, pp. 575–591
- ^ Weindruch, Richard (January 1996). "Calorie Restriction and Aging". Scientific American: 49–52.
- ^ Poovathingal SK, Gruber J, Halliwell B, Gunawan R (2009). "Stochastic drift in mitochondrial DNA point mutations: a novel perspective ex silico". PLOS Computational Biology. 5 (11): e1000572. Bibcode:2009PLSCB...5E0572P. doi:10.1371/journal.pcbi.1000572. PMC 2771766. PMID 19936024.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Conte D, Narindrasorasak S, Sarkar B (1996). "In vivo and in vitro iron-replaced zinc finger generates free radicals and causes DNA damage". The Journal of Biological Chemistry. 271 (9): 5125–30. doi:10.1074/jbc.271.9.5125. PMID 8617792.
- ^ a b c d Afanas'ev I. Signaling and Damaging Functions of Free Radicals in Aging-Free Radical Theory, Hormesis, and TOR. Aging And Disease. 2010;1(2) 75-88.
- ^ Pérez VI, Bokov A, Van Remmen H, Mele J, Ran Q, Ikeno Y, et al. (2009). "Is the oxidative stress theory of aging dead?". Biochimica et Biophysica Acta (BBA) - General Subjects. 1790 (10): 1005–14. doi:10.1016/j.bbagen.2009.06.003. PMC 2789432. PMID 19524016.
- ^ Yee C, Yang W, Hekimi S (2014). "The Intrinsic Apoptosis Pathway Mediates the Pro-Longevity Response to Mitochondrial ROS in C. elegans". Cell. 157 (4): 897–909. doi:10.1016/j.cell.2014.02.055. PMC 4454526. PMID 24813612.
- ^ a b c Brewer GJ (2010). "Epigenetic oxidative redox shift (EORS) theory of aging unifies the free radical and insulin signaling theories". Experimental Gerontology. 45 (3): 173–9. doi:10.1016/j.exger.2009.11.007. PMC 2826600. PMID 19945522.
- ^ a b Brink TC, Demetrius L, Lehrach H, Adjaye J (2009). "Age-related transcriptional changes in gene expression in different organs of mice support the metabolic stability theory of aging". Biogerontology. 10 (5): 549–64. doi:10.1007/s10522-008-9197-8. PMC 2730443. PMID 19031007.
- ^ Schulz TJ, Zarse K, Voigt A, Urban N, Birringer M, Ristow M (2007). "Glucose restriction extends Caenorhabditis elegans life span by inducing mitochondrial respiration and increasing oxidative stress". Cell Metabolism. 6 (4): 280–93. doi:10.1016/j.cmet.2007.08.011. PMID 17908557.
- ^ Sohal R, Mockett R, Orr W (2002). "Mechanisms of aging: an appraisal of the oxidative stress hypothesis". Free Radic Biol Med. 33 (5): 575–86. doi:10.1016/S0891-5849(02)00886-9. PMID 12208343.
- ^ Sohal R (2002). "Role of oxidative stress and protein oxidation in the aging process". Free Radic Biol Med. 33 (1): 37–44. doi:10.1016/S0891-5849(02)00856-0. PMID 12086680.
- ^ Rattan S (2006). "Theories of biological aging: genes, proteins, and free radicals". Free Radic Res. 40 (12): 1230–8. doi:10.1080/10715760600911303. PMID 17090411.
- ^ Bjelakovic G, Nikolova D, Gluud LL, Simonetti RG, Gluud C (2007). "Mortality in randomized trials of antioxidant supplements for primary and secondary prevention: systematic review and meta-analysis". The Journal of the American Medical Association. 297 (8): 842–57. doi:10.1001/jama.297.8.842. PMID 17327526.. See also the letter to JAMA by Philip Taylor and Sanford Dawsey and the reply by the authors of the original paper.
- ^ Castello L; Froio T; Cavallini G; Biasi F; Sapino A; Leonarduzzi G; et al. (2005). "Calorie restriction protects against age-related rat aorta sclerosis". FASEB Journal. 19 (13): 1863–5. doi:10.1096/fj.04-2864fje. PMID 16150801.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Ungvari Z, Parrado-Fernandez C, Csiszar A, de Cabo R. Mechanisms underlying caloric restriction and lifespan regulation: implications for vascular aging" Circulation Research 2008;102(5) 519-28.
- ^ a b Fontana L, Partridge L, Longo VD. Extending healthy life span--from yeast to humans. Science (New York, NY). 2010;328(5976) 321-6.
- ^ a b c Barja G. Mitochondrial oxygen consumption and reactive oxygen species production are independently modulated: implications for aging studies. Rejuvenation Research. 2007;10(2) 215-24.
- ^ a b Lewis KN, Andziak B, Yang T, Buffenstein R (2013). "The naked mole-rat response to oxidative stress: just deal with it". Antioxid. Redox Signal. 19 (12): 1388–99. doi:10.1089/ars.2012.4911. PMC 3791056. PMID 23025341.
- ^ a b MacRae SL, Croken MM, Calder RB, Aliper A, Milholland B, White RR, Zhavoronkov A, Gladyshev VN, Seluanov A, Gorbunova V, Zhang ZD, Vijg J (2015). "DNA repair in species with extreme lifespan differences". Aging. 7 (12): 1171–84. doi:10.18632/aging.100866. PMC 4712340. PMID 26729707.
- ^ a b Montgomery MK, Hulbert AJ, Buttemer WA (2012). "Does the oxidative stress theory of aging explain longevity differences in birds? I. Mitochondrial ROS production". Exp. Gerontol. 47 (3): 203–10. doi:10.1016/j.exger.2011.11.006. PMID 22123429.
External links
Calorie restriction
Biology of Aging
- Damage-Based Theories of Aging Includes a discussion of the free radical theory of aging.
