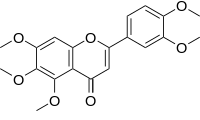
Sinensetin
| |

| |
| Names | |
|---|---|
| IUPAC name
2-(3,4-dimethoxyphenyl)-5,6,7-trimethoxychromen-4-one
| |
| Other names
3',4',5,6,7-pentamethoxy flavone
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.230.396 |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C20H20O7 | |
| Molar mass | 372.36 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Sinensetin is a methylated flavone. It can be found in Orthosiphon stamineus [1] and in orange oil. [2]
References
- ^ Akowuah, G.A.; Ismail, Z.; Norhayati, I.; Sadikun, A. (2005). "The effects of different extraction solvents of varying polarities on polyphenols of Orthosiphon stamineus and evaluation of the free radical-scavenging activity". Food Chemistry. 93 (2): 311. doi:10.1016/j.foodchem.2004.09.028.
- ^ Steinke, K., Jose, E., Sicker, D., Siehl, H.-U., Zeller, K.-P. and Berger, S. (2013), Sinensetin. Chemie in unserer Zeit, 47: 158–163. doi:10.1002/ciuz.201300627
