Diethylaluminium cyanide

| |

| |
| Names | |
|---|---|
| IUPAC name
diethylalumanylformonitrile
| |
| Other names
Cyanodiethyl Aluminum
(cyano-κC)diethyl-Aluminum (cyano-C)diethyl-Aluminum Cyanodiethyl-(7CI,8CI) Aluminum Cyanodiethylallane Cyanodiethylaluminum Diethylaluminum Cyanide | |
| Identifiers | |
| ECHA InfoCard | 100.024.873 |
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| Properties | |
| C 4H 10AlCN Et 2AlCN | |
| Molar mass | 111.12 g mol−1 |
| Appearance | dark brown, clear liquid (1.0 mol L−1 in toluene)[1] |
| Density | 0.864 g cm−3 at (25 °C) liquid |
| Boiling point | 162 °C (324 °F; 435 K) at 0.02 mmHg |
| Benzene, Toluene, diisopropyl ether | |
| Hazards | |
| Flash point | 7 °C (45 °F; 280 K) closed cup[1] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Diethylaluminum cyanide ("Nagata's reagent")[2] is the organoaluminum compound with formula ((C2H5)2AlCN)n. This colorless compound is usually handled as a solution in toluene. It is a reagent for the hydrocyanation of α,β-unsaturated ketones.[1][3][4][5][6]
Synthesis
Diethylaluminum cyanide was originally generated by treatment of triethylaluminum with a slight excess of hydrogen cyanide. The product is typically stored in ampoules because it is highly toxic. It dissolves in toluene, benzene, hexane and isopropyl ether. It undergoes hydrolysis readily and is not compatible with protic solvents.
- Et3Al + HCN → 1/n (Et2AlCN)n + EtH
Structure
Diethylaluminum cyanide has not been examined by X-ray crystallography, although other diorganoaluminum cyanides have been. Diorganylaluminum cyanides have the general formula (R2AlCN)n, and they exist as cyclic trimers (n = 3) or tetramers (n = 4). In these oligomers, one finds AlCN---Al linkages. One compound similar to diethylaluminum cyanide is bis[di(trimethylsilyl)methyl]aluminium cyanide, ((Me3Si)2CH)2AlCN, which has been shown crystallographically to exist as a trimer with the following structure:[4]
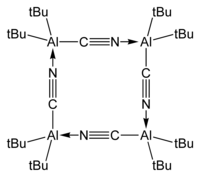
Bis(tert-butyl)aluminium cyanide, tBu2AlCN exists as a tetramer in the crystalline phase:[7][8]
Uses
Diethylaluminum cyanide is used for the stoichiometric hydrocyanation of α,β-unsaturated ketones. The reaction is influenced by the basicity of the solvent. This effect arises from the Lewis acidic qualities of the reagent.[9] The purpose of this reaction is to generate alkylnitriles, which are precursors to amines, amides, carboxylic acids esters and aldehydes.
References
- ^ a b c "MSDS - 276863". Sigma-Aldrich. Retrieved December 9, 2012.
- ^ Nagata, W (1988). "Diethylaluminum cyanide". Organic Syntheses. VI: 307. doi:10.15227/orgsyn.052.0090.
- ^ Nagata, W. (1966). "Alkylaluminum cyanides as potent reagents for hydrocyanation". Tetrahedron Lett. 7 (18): 1913–1918. doi:10.1016/S0040-4039(00)76271-X.
- ^ a b Uhl, Werner; Schütz, Uwe; Hiller, Wolfgang; Heckel, Maximilian (1995). "Synthese und Kristallstruktur des trimeren [(Me3Si)2CH]2Al—CN". Z. anorg. allg. Chem. 621 (5): 823–828. doi:10.1002/zaac.19956210521.
- ^ Wade, K.; Wyatt, B. K. (1969). "Reactions of organoaluminium compounds with cyanides. Part III. Reactions of trimethylaluminium, triethylaluminium, dimethylaluminium hydride, and diethylaluminium hydride with dimethylcyanamide". J. Chem. Soc.: 1121–1124. doi:10.1039/J19690001121.
- ^ Coates, G. E.; Mukherjee, R. N. (1963). "35. Dimethylaluminium cyanide and its gallium, indium, and thallium analogues; beryllium and methylberyllium cyanide". J. Chem. Soc.: 229–232. doi:10.1039/JR9630000229.
- ^ Uhl, W.; Matar, M. (2004). "Hydroalumination of nitriles and isonitriles" (PDF). Z. Naturforsch. B. 59 (11–12): 1214–1222.
- ^ Uhl, W.; Schütz, U.; Hiller, W.; Heckel, M. (2005). "Synthese und Kristallstruktur des trimeren [(Me3Si)2CH]2Al—CN" (PDF). Z. Naturforsch. B. 60 (2): 155–163.
- ^ Nagata, W.; Yoshioka, M. (1988). "Diethylaluminum cyanide". Organic Syntheses; Collected Volumes, vol. 6, p. 436.
External links
 Media related to Diethylaluminium cyanide at Wikimedia Commons
Media related to Diethylaluminium cyanide at Wikimedia Commons

![trimeric bis[di(trimethylsilyl)methyl]aluminium cyanide](http://upload.wikimedia.org/wikipedia/commons/thumb/8/8f/Cyclo-%28%28%28Me3Si%292CH%292AlCN%293-2D.png/300px-Cyclo-%28%28%28Me3Si%292CH%292AlCN%293-2D.png)