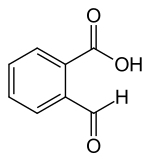
2-Carboxybenzaldehyde
This article may require cleanup to meet Wikipedia's quality standards. The specific problem is: Typography, demoting focus on synonyms and instead using consistent terminology, conversion from non-idiomatic translation and incorrect cite-template fields. (April 2019) |
| |
| Names | |
|---|---|
| Other names
2-formylbenzoic acid
2-phthaldehydic acid | |
| Identifiers | |
3D model (JSmol)
|
|
| 742381 | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.003.948 |
| EC Number |
|
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C8H6O3 | |
| soluble[1] | |
| Solubility | soluble in diethylether, ethanol[1] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
2-Carboxybenzaldehyde is a chemical compound. It consists of a benzene ring, with an aldehyde and a carboxylic acid as substituents that are ortho to each other. The compound exhibits ring–chain tautomerism: the two substituents can react with each other to form 3-hydroxyphthalide, a cyclic lactol. This lactol reacts readily with Grignard reagents , forming alkyl- and aryl-substituted phthalides.[2] Other benzo-fused heterocyclic compounds can be derived from 2-carboxybenzaldehyde, including isoindolinones and phthalazinones,[3] with a variety of pharmacological properties, such as the antihistamine azelastine.
Synthesis
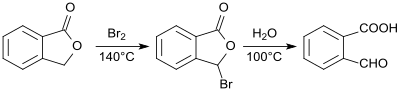
2-Carboxybenzaldehyde was first prepared from phthalide and characterized in 1887.[4] The reaction of bromine with phthalide produces 2-bromophthalide, which is converted into 2-formylbenzoic acid by heating with water in a total yield of 78 to 83%.[5]
An analogous process based on a chlorination reaction can also be used:[6]

The synthesis of 1-dichloromethyl-2-(trichloromethyl)benzene by photochlorination of o-xylene was also reported in 1887.[7]

The hydrolysis of the pentachloroxylene to the 2-carboxybenzaldehyde is carried out by boiling it with FeCl3-containing hydrochloric acid.[8]
In the reaction of phthalic anhydride with sodium tetracarbonylferrate, only one of the carboxyl groups is reduced to the aldehyde, the second remains unchanged.[9]
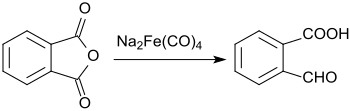
This gives 2-carboxybenzaldehyde in a yield of 61%.
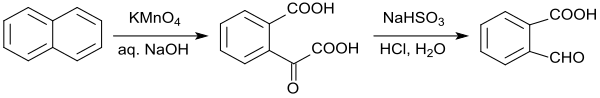
In a laboratory procedure, the oxidation of naphthalene with alkaline potassium permanganate is given, which, however, yields only a yield of 39% 2-carboxybenzaldehyde.[10] Also the oxidation of naphthalene with ozone to 2-formylbenzoic acid offers no significant advantages.[11]
Properties
Pure 2-carboxybenzaldehyde is a white crystalline powder which dissolves in water and in short-chain alcohols. In solid form and in most solvents, the substance is present as racemic 3-hydroxyphthalide (a lactol) as a result of ring–chain tautomerism.[8]

The refractive index is = 1.4500 (at 25 °C, 589 nm).[12]
Application
In the lactol form, 2-carboxybenzaldehyde behaves like a carboxylic acid anhydride and reacts smoothly with alcohols forming 3-alkoxyphthalides.[8]

Also with other nucleophilic compounds, such as, thiols, amines, amides, etc., 3-hydroxyphthalide reacts without a catalyst to give the corresponding derivatives.[8] For example, it reacts with morpholine in 91% yield to 3-morpholinyl phthalide.[13] 3-Hydroxyphthalide reacts with thionyl chloride at the hydroxyl group smoothly (80-90% yield) to 3-chlorophthalide.[13] With Grignard reagents, the hydroxy group can be exchanged for the corresponding alkyl or aryl group:[2]

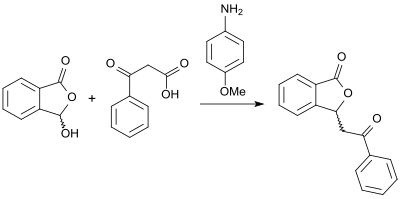
In the presence of (+)-cinchonine, in the reaction of (racemic) 3-hydroxyphthalide with carboxylic acid anhydrides to the corresponding chiral 3-substituted phthalides anenantiomeric excess of up to 90% ee can be achieved besides high product yields.[14] An alternative approach to (racemic) 3-substituted phthalides with high yields is opened up by the reaction of 2-carboxybenzaldehyde and β-keto acids in the presence of base 4-anisidine in glycerol as solvent.[15]
2-Carboxybenzaldehyde underdoes a double condensation reaction with hydrazine or alkylhydrazines, 1(2H)-phthalenazinones are obtained under acid catalysis with K10-montmorillonite and microwave irradiation in high yield.[16]
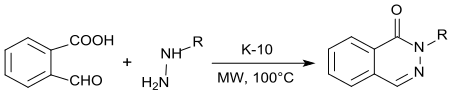
Phthalazinones (1(2H)-phthalenazinones) are important building blocks for natural products, fine chemicals and pharmaceutical active ingredients,[17] such as the antihypertensive vasodilator hydralazine.[6]
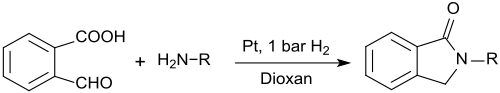
2-carboxybenzaldehyde can also be used for the preparation of N-substituted isoindolinones (1-isoindolinones, 2,3-dihydroindol-1-ones) which are formed when reacting 2-carboxybenzaldehyde with primary amines in the presence of platinum nanowires and low hydrogen pressure in 1,4-dioxane in very high yields:[17]
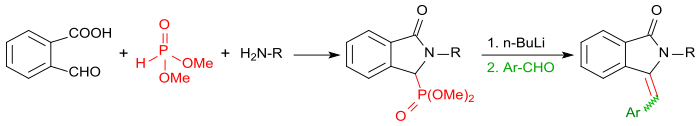
When the reaction of 2-carboxybenzaldehyde is carried out with primary amines in the presence of dimethylphosphite, the corresponding isoindolin-1-one-3-phosphonates are obtained first. After activation with butyllithium with aromatic aldehydes (such as, benzaldehyde in a Horner-Wadsworth-Emmons reaction), these can be converted in very high yields to 3-(arylmethylene)isoindolin-1-ones.[18]
More recently, 2-formylbenzoic acid became due to its reactivity of some interest as versatile molecular building block in multicomponent reactions, for example the Ugi reaction, for the synthesis of heterocyclic annellated aromatics. Functionalized isoindolinones are accessible in high yields in a three-component reaction with 2-formylbenzoic acid and 2-bromoanilines by palladium-catalyzed carbonylation.[19]

Another three-component reaction (here carried out as a Strecker synthesis) with 2-carboxybenzaldehyde, primary amines and potassium cyanide in methanol yields in the acidic medium an N-substituted isoindolinone-1-carbonitrile, formally an aminoacetonitrile derivative of isoindolinone with two moles of HCN.[20]

When substituted 2-formylbenzoic acid, potassium cyanide and equimolar amounts of primary aromatic amines and acetic acid are used, substituted isochromes-1-ones (isocumarins, 1H-2-benzopyran-1-ones) are obtained in good yields.[21]

The isochromenones obtained are quantitatively converted into isoindolinones by reacting in DMSO upon ring constriction into substituted isobenzofurans or with catalytic amounts of iodine in triethylamine.[22]
With isonitriles instead of potassium cyanide, 2-carboxybenzaldehyde and primary aromatic amines react in methanol to form substituted isochromen-1-ones, which are converted to isoindolinones with traces of acid.[23]

Synthesis pathways for the isoquinoline derivative[24] quinisocaine[25] (acting as a local anesthetic) and the antihistamine azelastine[26] are also based on 2-carboxybenzaldehyde as starting material.
References
- ^ a b William M. Haynes (2016), CRC Handbook of Chemistry and Physics, 97th Edition, Boca Raton, FL, U.S.A.: CRC Press, pp. 3–278, ISBN 978-1-4987-5429-3
- ^ a b P. Cannone; J. Plamondon; M. Akssira (1988), "Reactions selectives de organomagnesiens avec les lactols et les lactones. Synthese des diols primaires-secondaires", Tetrahedron, vol. 44, no. 10, pp. 2903–2912, doi:10.1016/S0040-4020(88)90027-0
- ^ V. Lad; J.G. Mulla; B.R. Agarwal; M. Farooqui (2015), "Nano TiO2: A recyclable catalyst for one pot synthesis of 2-(substituted phenyl)phthalazin-1(2H)-one" (PDF), J. Chem. Pharm. Res., vol. 7, no. 4, pp. 257–261
- ^ S. Racine (1887), "VIII. Ueber Phthalaldehydsäure" (PDF), Justus Liebigs Ann. Chem. (in German), vol. 239, no. 1, pp. 78–91, doi:10.1002/jlac.18872390106
- ^ "Phthalaldehydic acid". Organic Syntheses. doi:10.15227/orgsyn.023.0074.
- ^ a b J. Druey; B.H. Ringier (1951). "21. Hydrazinderivate der Phthalazin- und Pyridazinreihe". Helv. Chim. Acta (in German). 34 (1): 195–210. doi:10.1002/hlca.19510340122.
- ^ A. Colson; H. Gautier (1887), "Nouveau mode de chloruration des carbures", Ann. Chim. Phys., vol. 6, no. 11, pp. 19–32
- ^ a b c d D.D. Wheeler; D.C. Young; D.S. Erley (1957), "Reactions of phthalaldehydic acid", J. Org. Chem., vol. 22, no. 5, pp. 547–556, doi:10.1021/jo01356a022
- ^ Y. Watanabe; M. Yamashita; T-a. Mitsudo; M. Tanaka; Y. Takegami (1973), "The facile synthesis of aldehydes and aldehydic acids from carboxylic acid anhydrides using sodium tetracarbonylferrate", Tetrahedron Lett., vol. 14, no. 37, pp. 3535–3536, doi:10.1016/S0040-4039(01)86963-X
- ^ "Phthalaldehydic acid". Organic Syntheses. doi:10.15227/orgsyn.016.0068.
- ^ L. Seekles (1924), "Ortho phthalaldehydic acid", Rec. Trav. Chim., vol. 43, no. 5, pp. 329–340, doi:10.1002/recl.19240430506
- ^ Carl L. Yaws (2015), Thermophysical Properties of Chemicals and Hydrocarbons, 2nd Edition, Oxford, UK: Elsevier Inc., p. 183, ISBN 978-0-323-28659-6
- ^ a b K.B. Sloan; S.A.M. Koch (1983), "Effect of Nucleophilicity and Leaving Group Ability on the SN2 Reactions of Amines with (Acyloxy)alkyl α-Halides: A Product Distribution Study", J. Org. Chem., vol. 48, no. 5, pp. 635–640, doi:10.1021/jo00153a002
- ^ D. Niedek; S.M.M. Schuler; C. Eschmann; R.C. Wende; A. Seitz; F. Keul; P.R. Schreiner (2017), "Synthesis of enantioenriched phthalides and isoindolinone derivatives from 2-formylbenzoic acid", Synthesis, vol. 49, no. 02, pp. 371–382, doi:10.1055/s0036-1589404
- ^ L. Jia; F. Han (2017), "Sustainable synthesis of 3-substituted phthalides via a catalytic one-pot cascade strategy from 2-formylbenzoic acid with β-keto acids in glycerol", Beilstein J. Org. Chem., vol. 13, pp. 1425–1429, doi:10.3762/bjoc.13.139, PMC 5530723, PMID 28781708
- ^ V.M. Outerbridge; S.M. Landge; H. Tamaki; B. Török (2009), "Microwave-promoted solid-acid-catalyzed one-pot synthesis of phthalazinones", Synthesis, vol. 11, pp. 1801–1806, doi:10.1055/s-0028-1088074
- ^ a b L. Shi; L. Hu; J. Wang; X. Cao; H. Gu (2012), "Highly efficient synthesis of N-substituted isoindolinones and phthalazinones using Pt nanowires as catalysts", Org. Lett., vol. 14, no. 7, pp. 1876–1879, doi:10.1021/ol300471a
- ^ M.A. Reyes-Gonzàlez; A. Zamundio-Medina; M. Ordónez (2012), "Practical and highly selective synthesis of 3-(arylmethylene)isoindolin-1-ones from 2-formylbenzoic acid", Tetrahedron Lett., vol. 53, no. 43, pp. 5756–5758, doi:10.1016/j.tetlet.2012.08.040
- ^ K. Natte; J. Chen; H. Li; H. Neumann; M. Beller; X.F. Wu (2014), "Palladium-catalyzed carbonylation of 2-bromoanilines with 2-formylbenzoic acid and 2-halobenzaldehydes: Efficient synthesis of functionalized isoindolinones", Chem. Eur. J., vol. 20, no. 44, pp. 14184–14188, doi:10.1002/chem.201404446
- ^ T. Opatz; D. Ferenc (2004), "An unexpected three-component condensation leading to amino-(3-oxo-2,3-dihydro-1H-isoindol-1-ylidene)-acetonitriles", J. Org. Chem., vol. 69, no. 24, pp. 8496–8499, doi:10.1021/jo0486802
- ^ T. Opatz; D. Ferenc (2005), "Facile preparation of 3-amino-4-(arylamino)-1H-isochromen-1-ones by a new multi-component reaction", Eur. J. Org. Chem., vol. 5, pp. 817–821, doi:10.1002/ejoc.200400685
- ^ T. Opatz; D. Ferenc (2006), "Ring contracting rearrangements of 3-amino-4-(arylamino)-1H-isochromen-1-ones", Eur. J. Org. Chem., vol. 1, pp. 121–126, doi:10.1002/ejoc.200500575
- ^ C. Faggi; M. Garcia-Valverde; S. Macaccini; G. Menchi (2010), "Isolation of Ugi four-component condensation primary adducts: A straightforward route to isocoumarins", Org. Lett., vol. 12, no. 4, pp. 788–791, doi:10.1021/ol9028622
- ^ J.W. Wilson; N.D. Dawson; W. Brooks; G.E. Ullyot (1949), "Local anesthetics. Aminoalkoxyisoquinoline derivatives", J. Am. Chem. Soc., vol. 71, no. 3, pp. 937–938, doi:10.1002/ja01171a047
- ^ F. v. Bruchhausen; et al., eds. (1994), Hagers Handbuch der Pharmazeutischen Praxis, 5. Aufl. (in German), Berlin: Springer, p. 482, doi:10.1007/978-3-642-57880-9, ISBN 978-3-642-63389-8
- ^ G. Scheffler; J. Engel; B. Kutscher; W.S. Sheldrick; P. Bell (1988), "Synthese und Kristallstrukturanalyse von Azelastin", Arch. Pharm. (in German), vol. 321, no. 4, pp. 205–208, doi:10.1002/ardp.19883210406
