L-form bacteria

L-form bacteria, also known as L-phase bacteria, L-phase variants, and cell wall-deficient (CWD) bacteria, are strains of bacteria that lack cell walls.[1] They were first isolated in 1935 by Emmy Klieneberger-Nobel, who named them "L-forms" after the Lister Institute in London where she was working.[2]
Two types of L-forms are distinguished: unstable L-forms, spheroplasts that are capable of dividing, but can revert to the original morphology, and stable L-forms, L-forms that are unable to revert to the original bacteria.
Some parasitic species of bacteria, such as mycoplasma, also lack a cell wall,[3] but these are not considered L-forms since they are not derived from bacteria that normally have cell walls.[4]
Appearance and cell division
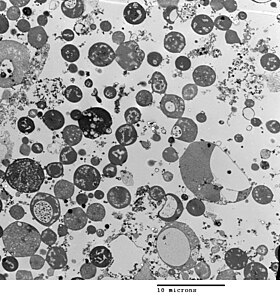
Bacterial morphology is determined by the cell wall. Since the L-form has no cell wall, its morphology is different from that of the strain of bacteria from which it is derived. Typical L-form cells are spheres or spheroids. For example, L-forms of the rod-shaped bacterium Bacillus subtilis appear round when viewed by phase contrast microscopy or by transmission electron microscopy.[5]
Although L-forms can develop from Gram-positive as well as from Gram-negative bacteria, in a Gram stain test, the L-forms always colour Gram-negative, due to the lack of a cell wall.
The cell wall is important for cell division, which, in most bacteria, occurs by binary fission. This process usually requires a cell wall and components of the bacterial cytoskeleton such as FtsZ. The ability of L-form bacteria to grow and divide in the absence of both of these structures is highly unusual, and may represent a form of cell division that was important in early forms of life. This novel mode of division seems to involve the extension of thin protrusions from the cell's surface and these protrusions then pinching off to form new cells. The lack of cell wall in L-forms means that division is disorganised, giving rise to a variety of cell sizes, from very tiny to very big.[1]
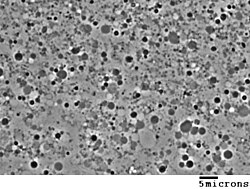
Generation in cultures
L-forms can be generated in the laboratory from many bacterial species that usually have cell walls, such as Bacillus subtilis or Escherichia coli. This is done by inhibiting peptidoglycan synthesis with antibiotics or treating the cells with lysozyme, an enzyme that digests cell walls. The L-forms are generated in a culture medium that is the same osmolarity as the bacterial cytosol (an isotonic solution), which prevents cell lysis by osmotic shock.[2] L-form strains can be unstable, tending to revert to the normal form of the bacteria by regrowing a cell wall, but this can be prevented by long-term culture of the cells under the same conditions that were used to produce them – letting the wall-disabling mutations to accumulate by genetic drift.[6]
Some studies have identified mutations that occur, as these strains are derived from normal bacteria.[1][2] One such point mutation D92E is in an enzyme yqiD/ispA (P54383) involved in the mevalonate pathway of lipid metabolism that increased the frequency of L-form formation 1,000-fold.[1] The reason for this effect is not known, but it is presumed that the increase is related to this enzyme's role in making a lipid important in peptidoglycan synthesis.
Another methodology of induction relies on nanotechnology and landscape ecology. Microfluidics devices can be built in order to challenge peptidoglycan synthesis by extreme spatial confinement. After biological dispersal through a constricted (sub-micrometre scale) biological corridor connecting adjacent micro habitat patches, L-form-like cells can be derived[7] using a microfluifics-based (synthetic) ecosystem implementing an adaptive landscape[8] selecting for shape-shifting phenotypes similar to L-forms.
Significance and applications
Some publications have suggested that L-form bacteria might cause diseases in humans,[9] and other animals[10] but, as the evidence that links these organisms to disease is fragmentary and frequently contradictory, this hypothesis remains controversial.[11][12] The two extreme viewpoints on this question are that L-form bacteria are either laboratory curiosities of no clinical significance or important but unappreciated causes of disease.[4] Research on L-form bacteria is continuing. For example, L-form organisms have been observed in mouse lungs after experimental inoculation with Nocardia caviae,[13][14] and a recent study suggested that these organisms may infect immunosuppressed patients having undergone bone marrow transplants.[15] The formation of strains of bacteria lacking cell walls has also been proposed to be important in the acquisition of bacterial antibiotic resistance.[16][17]
L-form bacteria may be useful in research on early forms of life, and in biotechnology. These strains are being examined for possible uses in biotechnology as host strains for recombinant protein production.[18][19][20] Here, the absence of a cell wall can allow production of large amounts of secreted proteins that would otherwise accumulate in the periplasmic space of bacteria.[21][22]
See also
- Mycoplasmataceae—lack peptidoglycan but supplement their membranes with sterols for stability.
- Protoplast
- Spheroplast
- Ultramicrobacteria
References
- ^ a b c d Leaver M, Domínguez-Cuevas P, Coxhead JM, Daniel RA, Errington J (February 2009). "Life without a wall or division machine in Bacillus subtilis". Nature. 457 (7231): 849–53. Bibcode:2009Natur.457..849L. doi:10.1038/nature07742. PMID 19212404.
- ^ a b c Joseleau-Petit D, Liébart JC, Ayala JA, D'Ari R (September 2007). "Unstable Escherichia coli L Forms Revisited: Growth Requires Peptidoglycan Synthesis". J. Bacteriol. 189 (18): 6512–20. doi:10.1128/JB.00273-07. PMC 2045188. PMID 17586646.
- ^ Razin S, Yogev D, Naot Y (December 1998). "Molecular Biology and Pathogenicity of Mycoplasmas". Microbiol. Mol. Biol. Rev. 62 (4): 1094–156. doi:10.1128/MMBR.62.4.1094-1156.1998. PMC 98941. PMID 9841667.
- ^ a b Domingue GJ, Woody HB (April 1997). "Bacterial persistence and expression of disease". Clin. Microbiol. Rev. 10 (2): 320–44. doi:10.1128/CMR.10.2.320. PMC 172922. PMID 9105757. Full PDF
- ^ Gilpin RW, Young FE, Chatterjee AN (January 1973). "Characterization of a Stable L-Form of Bacillus subtilis 168". J. Bacteriol. 113 (1): 486–99. doi:10.1128/JB.113.1.486-499.1973. PMC 251652. PMID 4631836.
- ^ Allan EJ (April 1991). "Induction and cultivation of a stable L-form of Bacillus subtilis". J. Appl. Bacteriol. 70 (4): 339–43. doi:10.1111/j.1365-2672.1991.tb02946.x. PMID 1905284.
- ^ Männik J.; R. Driessen; P. Galajda; J.E. Keymer; C. Dekker (September 2009). "Bacterial growth and motility in sub-micron constrictions". PNAS. 106 (35): 14861–14866. Bibcode:2009PNAS..10614861M. doi:10.1073/pnas.0907542106. PMC 2729279. PMID 19706420.
- ^ Keymer J.E.; P. Galajda; C. Muldoon R.; R. Austin (November 2006). "Bacterial metapopulations in nanofabricated landscapes". PNAS. 103 (46): 17290–295. Bibcode:2006PNAS..10317290K. doi:10.1073/pnas.0607971103. PMC 1635019. PMID 17090676.
- ^ Wall S, Kunze ZM, Saboor S, Soufleri I, Seechurn P, Chiodini R, McFadden JJ (1993). "Identification of spheroplast-like agents isolated from tissues of patients with Crohn's disease and control tissues by polymerase chain reaction". J. Clin. Microbiol. 31 (5): 1241–5. doi:10.1128/JCM.31.5.1241-1245.1993. PMC 262911. PMID 8501224.
- ^ Hulten K, Karttunen TJ, El-Zimaity HM, Naser SA, Collins MT, Graham DY, El-Zaatari FA (2000). "Identification of cell wall deficient forms of M. avium subsp. paratuberculosis in paraffin embedded tissues from animals with Johne's disease by in situ hybridization". J. Microbiol. Methods. 42 (2): 185–95. doi:10.1016/S0167-7012(00)00185-8. PMID 11018275.
- ^ Onwuamaegbu ME, Belcher RA, Soare C (2005). "Cell wall-deficient bacteria as a cause of infections: a review of the clinical significance" (PDF). J. Int. Med. Res. 33 (1): 1–20. doi:10.1177/147323000503300101. PMID 15651712. Archived from the original (PDF) on 24 August 2009.
- ^ Casadesús J (December 2007). "Bacterial L-forms require peptidoglycan synthesis for cell division". BioEssays. 29 (12): 1189–91. doi:10.1002/bies.20680. PMID 18008373.
- ^ Beaman BL (July 1980). "Induction of L-phase variants of Nocardia caviae within intact murine lungs". Infect. Immun. 29 (1): 244–51. doi:10.1128/IAI.29.1.244-251.1980. PMC 551102. PMID 7399704.
- ^ Beaman BL, Scates SM (September 1981). "Role of L-forms of Nocardia caviae in the development of chronic mycetomas in normal and immunodeficient murine models". Infect. Immun. 33 (3): 893–907. doi:10.1128/IAI.33.3.893-907.1981. PMC 350795. PMID 7287189.
- ^ Woo PC, Wong SS, Lum PN, Hui WT, Yuen KY (March 2001). "Cell-wall-deficient bacteria and culture-negative febrile episodes in bone-marrow-transplant recipients". Lancet. 357 (9257): 675–9. doi:10.1016/S0140-6736(00)04131-3. PMID 11247551.
- ^ Fuller E, Elmer C, Nattress F, et al. (December 2005). "β-Lactam Resistance in Staphylococcus aureus Cells That Do Not Require a Cell Wall for Integrity". Antimicrob. Agents Chemother. 49 (12): 5075–80. doi:10.1128/AAC.49.12.5075-5080.2005. PMC 1315936. PMID 16304175.
- ^ Possible role of L-form switching in recurrent urinary tract infection Nature, 2019
- ^ Sieben, Stefan (April 1998). "Die stabilen Protoplasten-Typ L-Formen von Proteus mirabilis als neues Expressionssystem für sekretorische Proteine und integrale Mempranproteine". Dissertation Universität Jena. OCLC 246350676.
- ^ Sieben S, Hertle R, Gumpert J, Braun V (October 1998). "The Serratia marcescens hemolysin is secreted but not activated by stable protoplast-type L-forms of Proteus mirabilis". Arch. Microbiol. 170 (4): 236–42. doi:10.1007/s002030050638. PMID 9732437.
- ^ Gumpert J, Hoischen C (October 1998). "Use of cell wall-less bacteria (L-forms) for efficient expression and secretion of heterologous gene products". Current Opinion in Biotechnology. 9 (5): 506–9. doi:10.1016/S0958-1669(98)80037-2. PMID 9821280.
- ^ Rippmann JF, Klein M, Hoischen C, et al. (1 December 1998). "Procaryotic Expression of Single-Chain Variable-Fragment (scFv) Antibodies: Secretion in L-Form Cells of Proteus mirabilis Leads to Active Product and Overcomes the Limitations of Periplasmic Expression in Escherichia coli". Appl. Environ. Microbiol. 64 (12): 4862–9. doi:10.1128/AEM.64.12.4862-4869.1998. PMC 90935. PMID 9835575.
- ^ Choi JH, Lee SY (June 2004). "Secretory and extracellular production of recombinant proteins using Escherichia coli". Appl. Microbiol. Biotechnol. 64 (5): 625–35. doi:10.1007/s00253-004-1559-9. PMID 14966662.
Further reading
- Domingue, Gerald J. (1982). Cell wall-deficient bacteria: basic principles and clinical significance. Reading, Mass: Addison-Wesley Pub. Co. ISBN 978-0-201-10162-1.
- Mattman, Lida H. (2001). Cell wall deficient forms: stealth pathogens. Boca Raton: CRC. ISBN 978-0-8493-8767-8.
External links
- Errington Group at Newcastle University
- Scientists explore new window on the origins of life 2009 Newcastle University press release
