Trifarotene
Appearance
 | |
| Clinical data | |
|---|---|
| Trade names | Aklief |
| Other names | CD5789 |
| AHFS/Drugs.com | Monograph |
| License data |
|
| Routes of administration | Topical |
| Drug class | Skin and mucous membrane agents |
| ATC code | |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.278.901 |
| Chemical and physical data | |
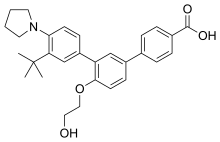
| Formula | C29H33NO4 |
| Molar mass | 459.586 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Trifarotene, sold under the brand name Aklief, is a drug for the topical treatment of acne vulgaris in those nine years of age and older.[1] It is a retinoid;[2] more specifically, it is a fourth generation selective retinoic acid receptor (RAR)-γ agonist.[3]
It was approved for medical use in the United States in 2019,[1][4][5] but is not approved in the European Union as of June 2020[update].[6]
References
- ^ a b "Drug Trials Snapshots: Aklief". U.S. Food and Drug Administration (FDA). 11 October 2019. Archived from the original on 19 November 2019. Retrieved 18 November 2019.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ^ Trifarotene Monograph
- ^ Scott, Lesley J. (2019). "Trifarotene: First Approval". Drugs. 79 (17): 1905–1909. doi:10.1007/s40265-019-01218-6. ISSN 0012-6667.
- ^ "Aklief (trifarotene) FDA Approval History". Drugs.com. 7 October 2019. Retrieved 19 November 2019.
- ^ "Drug Approval Package: Aklief". U.S. Food and Drug Administration (FDA). 21 October 2019. Archived from the original on 19 November 2019. Retrieved 18 November 2019.
- ^ "Trifarotene". European Medicines Agency. Retrieved 17 June 2020.
External links
- "Trifarotene". Drug Information Portal. U.S. National Library of Medicine (NLM).
