Zinc azide
Appearance
| |
| Names | |
|---|---|
| IUPAC name
Zinc(II) azide
| |
| Other names
Zinc diazide
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| Zn(N3)2 | |
| Molar mass | 149.4 g/mol |
| Appearance | white solid |
| Density | 2.559 g/cm3 (alpha polymorph) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
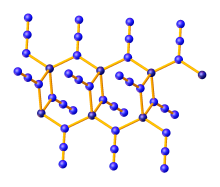
Zinc azide (Zn(N3)2) is an inorganic compound composed of zinc and azide. It is a white, explosive solid. It is a coordination polymer, which crystallizes in three polymorphs, all of which feature tetrahedral zinc centers and bridging azide ligands. They are prepared by the protonolysis of diethyl zinc with hydrazoic acid:[1]
- Zn(C2H5)2 + 2 HN3 → Zn(N3)2 + 2 C2H6
References
- ^ Schultz, Axel; Villanger, Alexander (2016). "Binary Zinc Azides". Chemistry: A European Journal. 22: 2032–2038. doi:10.1002/chem.201504524.
