From Wikipedia, the free encyclopedia
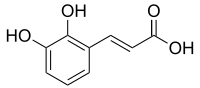
2,3-Dihydroxycinnamic acid
|
| Names
|
| IUPAC name
(E)-3-(2,3-Dihydroxyphenyl)prop-2-enoic acid
|
| Other names
trans-2,3-Dihydroxycinnamate
3-(2,3-Dihydroxyphenyl)acrylic acid
|
| Identifiers
|
|
|
|
|
|
|
| ChemSpider
|
|
|
|
|
| UNII
|
|
|
|
|
InChI=1S/C9H8O4/c10-7-3-1-2-6(9(7)13)4-5-8(11)12/h1-5,10,13H,(H,11,12)/b5-4+ Key: SIUKXCMDYPYCLH-SNAWJCMRSA-N InChI=1/C9H8O4/c10-7-3-1-2-6(9(7)13)4-5-8(11)12/h1-5,10,13H,(H,11,12)/b5-4+ Key: SIUKXCMDYPYCLH-SNAWJCMRBO
|
c1cc(c(c(c1)O)O)/C=C/C(=O)O
|
| Properties
|
|
|
C9H8O4
|
| Molar mass
|
180.159 g·mol−1
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). |
Chemical compound
2,3-Dihydroxycinnamic acid is a hydroxycinnamic acid. It is an isomer of caffeic acid.
It is a metabolite found in human urine.[1]
References
- ^ Heindl, A; Rau, O; Spiteller, G (1985). "Identification of aromatic dihydroxy acids in biological fluids". Biomedical Mass Spectrometry. 12 (2): 59–66. doi:10.1002/bms.1200120203. PMID 3158357.
|
|---|
| Aglycones | | Precursor | |
|---|
Monohydroxycinnamic acids
(Coumaric acids) | |
|---|
| Dihydroxycinnamic acids | |
|---|
| Trihydroxycinnamic acids | |
|---|
| O-methylated forms | |
|---|
| others | |
|---|
|
|---|
| Esters | | glycoside-likes | Esters of
caffeic acid
with cyclitols | |
|---|
| Glycosides | |
|---|
|
|---|
| Tartaric acid esters | |
|---|
Other esters
with caffeic acid | |
|---|
Caffeoyl phenylethanoid
glycoside (CPG) |
- Echinacoside
- Calceolarioside A, B, C, F
- Chiritoside A, B, C
- Cistanoside A, B, C, D, E, F, G, H
- Conandroside
- Myconoside
- Pauoifloside
- Plantainoside A
- Plantamajoside
- Tubuloside B
- Verbascoside (Isoverbascoside, 2′-Acetylverbascoside)
|
|---|
|
|---|
| Oligomeric forms | | Dimers |
- Diferulic acids (DiFA) : 5,5′-Diferulic acid, 8-O-4′-Diferulic acid, 8,5′-Diferulic acid, 8,5′-DiFA (DC), 8,5′-DiFA (BF), 8,8′-Diferulic acid
|
|---|
| Trimers | |
|---|
| Tetramers | |
|---|
|
|---|
Conjugates with
coenzyme A (CoA) | |
|---|

