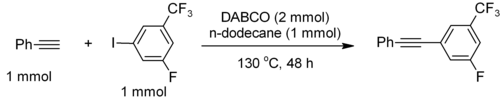DABCO
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
1,4-Diazabicyclo[2.2.2]octane
| |||
| Other names
Triethylenediamine, TEDA
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.005.455 | ||
| EC Number |
| ||
PubChem CID
|
|||
| UNII | |||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| C6H12N2 | |||
| Molar mass | 112.176 g·mol−1 | ||
| Appearance | White crystalline powder | ||
| Melting point | 156 to 160 °C (313 to 320 °F; 429 to 433 K) | ||
| Boiling point | 174 °C (345 °F; 447 K) | ||
| Soluble, hygroscopic | |||
| Acidity (pKa) | 3.0, 8.8 (in water)[1] | ||
| Hazards | |||
| Occupational safety and health (OHS/OSH): | |||
Main hazards
|
Harmful | ||
| GHS labelling: | |||
  
| |||
| Danger | |||
| H228, H302, H315, H319, H335, H412 | |||
| P210, P261, P273, P305+P351+P338 | |||
| NFPA 704 (fire diamond) | |||
| Flash point | 62 °C (144 °F; 335 K) | ||
| Related compounds | |||
Related compounds
|
Quinuclidine Tropane | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
DABCO (1,4-diazabicyclo[2.2.2]octane) is a bicyclic organic compound with the formula N2(C2H4)3. This colorless solid is a highly nucleophilic tertiary amine base, which is used as a catalyst and reagent in polymerization and organic synthesis.[3]
Quinuclidine has a similar structure, with one of the nitrogen atoms replaced by a carbon atom.
Reactions and applications
The pKa of [HDABCO]+ (the protonated derivative) is 8.8, which is almost the same as ordinary alkylamines. The nucleophilicity of the amine is high because the amine centers are unhindered. It is sufficiently basic to promote C–C coupling of terminal acetylenes, for example, phenylacetylene couples with electron-deficient iodoarenes.
Catalyst
DABCO is used as a base-catalyst for:
- formation of polyurethane from alcohol and isocyanate functionalized monomers and pre-polymers.[4]
- Baylis-Hillman and Morita-Baylis-Hillman reactions of aldehydes and unsaturated ketones and aldehydes.[5]
Lewis base
As an unhindered amine, it is a strong ligand and Lewis base. It forms a crystalline 2:1 adduct with hydrogen peroxide[6] and sulfur dioxide.[7]
Ionic monomer synthesis
DABCO can be used to synthesize doubly-charged styrenic monomers. These ionic monomers allow synthesis of polyelectrolytes and ionomers with two cyclic quaternary ammonium cations on each ionic pendant group. [8]
Quencher of singlet oxygen
DABCO and related amines are quenchers of singlet oxygen and effective antioxidants,[9] and can be used to improve the lifetime of dyes. This makes DABCO useful in dye lasers and in mounting samples for fluorescence microscopy (when used with glycerol and PBS).[10] DABCO can also be used to demethylate quaternary ammonium salts by heating in dimethylformamide (DMF).[11]
Production
It is produced by thermal reactions of compounds of the type H2NCH2CH2X (X = OH, NH2, or NHR) in the presence of zeolitic catalysts. An idealized conversion is shown for the conversion from ethanolamine:[12]
- 3 H2NCH2CH2OH → N(CH2CH2)3N + NH3 + 3 H2O
References
- ^ D. H. Ripin; D. A. Evans (2002). "pKa's of Nitrogen Acids" (PDF).
- ^ "Safety data for 1,4-diazabicyclo[2.2.2]octane (see MSDS)". Sigma-Aldrich.
- ^ Uppuluri V. Mallavadhani, Nicolas Fleury-Bregeot. "1,4-Diazabicyclo [2.2.2]octane". In Encyclopedia of Reagents for Organic Synthesis, 2010, John Wiley & Sons, Ltd. doi:10.1002/047084289X.rd010m.pub2
- ^ "Polyurethane additives guide" (PDF). Air Products & Chemicals. Archived from the original (PDF) on 2016-03-06.
- ^ Baylis, A. B.; Hillman, M. E. D. German Patent 2155113, 1972.
- ^ P. Dembech, A. Ricci, G. Seconi, and M. Taddei "Bis(trimethylsilyl) Peroxide" Org. Synth. 1997, volume 74, pp. 84. doi:10.15227/orgsyn.074.0084
- ^ Ludovic Martial and Laurent Bischoff "Preparation of DABSO from Karl-Fischer Reagent" Org. Synth. 2013, volume 90, pp. 301. doi:10.15227/orgsyn.090.0301
- ^ Zhang, K.; Drummey, K. J.; Moon, N. G.; Chiang, W. D.; Long, T. E. (2016). "Styrenic DABCO salt-containing monomers for the synthesis of novel charged polymers". Polymer Chemistry. 7 (20): 3370–3374. doi:10.1039/C6PY00426A.
- ^ Ouannes, C.; Wilson, T. (1968). "Quenching of singlet oxygen by tertiary aliphatic amines. Effect of DABCO (1,4-diazabicyclo[2.2.2]octane)". Journal of the American Chemical Society. 90 (23): 6527–6528. doi:10.1021/ja01025a059.
- ^ Valnes, K.; Brandtzaeg, P. (1985). "Retardation of immunofluorescence fading during microscopy" (PDF). Journal of Histochemistry and Cytochemistry. 33 (8): 755–761. doi:10.1177/33.8.3926864. PMID 3926864.
- ^ Ho, T. L. (1972). "Dealkylation of Quaternary Ammonium Salts with 1,4-Diazabicyclo[2.2.2]octane". Synthesis. 1972 (12): 702. doi:10.1055/s-1972-21977.
- ^ Karsten Eller, Erhard Henkes, Roland Rossbacher, Hartmut Höke "Amines, Aliphatic" in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim, 2005. doi:10.1002/14356007.a02_001
Further reading
- Cecchi, L.; DeSarlo, F.; Machetti, F. (2006). "1,4-Diazabicyclo[2.2.2]octane (DABCO) as an Efficient Reagent for the Synthesis of Isoxazole Derivatives from Primary Nitro Compounds and Dipolarophiles: The Role of the Base". European Journal of Organic Chemistry. 2006 (21): 4852–4860. doi:10.1002/ejoc.200600475..