Boron trioxide

| |
| |
| Names | |
|---|---|
| Other names
boron oxide, diboron trioxide, boron sesquioxide, boric oxide, boria
Boric acid anhydride | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.013.751 |
PubChem CID
|
|
| RTECS number |
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| B2O3 | |
| Molar mass | 69.6182 g/mol |
| Appearance | white, glassy solid |
| Density | 2.460 g/cm3, liquid; 2.55 g/cm3, trigonal; |
| Melting point | 450 °C (trigonal) 510 °C (tetrahedral) |
| Boiling point | 1860 °C,[1] sublimates at 1500 °C[2] |
| 22 g/L | |
| Solubility | partially soluble in methanol |
| Acidity (pKa) | ~ 4 |
| Hazards | |
| NFPA 704 (fire diamond) | |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose)
|
3150 mg/kg (oral, rat) |
| Supplementary data page | |
| Boron trioxide (data page) | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Boron trioxide (or diboron trioxide) is one of the oxides of boron. It is a white, glassy solid with the formula B2O3. It is almost always found as the vitreous (amorphic) form; however, it can be crystallized after extensive annealing. It is one of the most difficult compounds known to crystallize.[clarification needed]
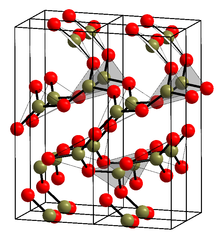
Glassy boron oxide (g-B2O3) is thought to be composed of boroxol rings which are six-membered rings composed of alternating 3-coordinate boron and 2-coordinate oxygen. This view is controversial, however, because no model has ever been made of glassy boron oxide of the correct density containing a large number of six-membered rings. The rings are thought to make a few BO3 triangles, but mostly link (polymerize) into ribbons and sheets.[4][5] The crystalline form (α-B2O3) (see structure in the infobox[3]) is exclusively composed of BO3 triangles. This trigonal, quartz-like network undergoes a coesite-like transformation to monoclinic β-B2O3 at several gigapascals (9.5 GPa).[6]
Preparation
Boron trioxide is produced by treating borax with sulfuric acid in a fusion furnace. At temperatures above 750 °C, the molten boron oxide layer separates out from sodium sulfate. It is then decanted, cooled and obtained in 96–97% purity.[2]
Another method is heating boric acid above ~300 °C. Boric acid will initially decompose into water steam and metaboric acid (HBO2) at around 170 °C, and further heating above 300 °C will produce more steam and boron trioxide. The reactions are:
- H3BO3 → HBO2 + H2O
- 2 HBO2 → B2O3 + H2O
Boric acid goes to anhydrous microcrystalline B2O3 in a heated fluidized bed.[7] Carefully controlled heating rate avoids gumming as water evolves. Molten boron oxide attacks silicates. Internally graphitized tubes via acetylene thermal decomposition are passivated.[8]
Crystallization of molten α-B2O3 at ambient pressure is strongly kinetically disfavored (compare liquid and crystal densities). Threshold conditions for crystallization of the amorphous solid are 10 kbar and ~200 °C.[9] Its proposed crystal structure in enantiomorphic space groups P31(#144); P32(#145)[10][11] (e.g., γ-glycine) has been revised to enantiomorphic space groups P3121(#152); P3221(#154)[12](e.g., α-quartz).
Hardness
The bulk modulus of β-B2O3 is rather high (K = 180 GPa). The Vickers hardness of g-B2O3 is 1.5 GPa and of β-B2O3 is 16 GPa.[13]
Applications
- Fluxing agent for glass and enamels
- Starting material for synthesizing other boron compounds such as boron carbide
- An additive used in glass fibres (optical fibres)
- It is used in the production of borosilicate glass
- The inert capping layer in the LEC process for the production of gallium arsenide single crystal
- As an acid catalyst in organic synthesis
See also
References
- ^ High temperature corrosion and materials chemistry: proceedings of the Per Kofstad Memorial Symposium. Proceedings of the Electrochemical Society. The Electrochemical Society. 2000. p. 496. ISBN 1-56677-261-3.
- ^ a b Patnaik, P. (2003). Handbook of Inorganic Chemical Compounds. McGraw-Hill. p. 119. ISBN 0-07-049439-8. Retrieved 2009-06-06.
- ^ a b Gurr, G. E.; Montgomery, P. W.; Knutson, C. D.; Gorres, B. T. (1970). "The Crystal Structure of Trigonal Diboron Trioxide". Acta Crystallographica B. 26 (7): 906–915. doi:10.1107/S0567740870003369.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Eckert, H. (1992). "Structural characterization of noncrystalline solids and glasses using solid state NMR". Progress in Nuclear Magnetic Resonance Spectroscopy. 24 (3): 159–293. doi:10.1016/0079-6565(92)80001-V.
- ^ Hwang, S.-J.; Fernandez, C.; Amoureux, J. P.; Cho, J.; Martin, S. W.; Pruski, M. (1997). "Quantitative study of the short range order in B2O3 and B2S3 by MAS and two-dimensional triple-quantum MAS 11B NMR". Solid State Nuclear Magnetic Resonance. 8 (2): 109–121. doi:10.1016/S0926-2040(96)01280-5. PMID 9203284.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Brazhkin, V. V.; Katayama, Y.; Inamura, Y.; Kondrin, M. V.; Lyapin, A. G.; Popova, S. V.; Voloshin, R. N. (2003). "Structural transformations in liquid, crystalline and glassy B2O3 under high pressure". JETP Letters. 78 (6): 393–397. doi:10.1134/1.1630134.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Kocakuşak, S.; Akçay, K.; Ayok, T.; Koöroğlu, H. J.; Koral, M.; Savaşçi, Ö. T.; Tolun, R. (1996). "Production of anhydrous, crystalline boron oxide in fluidized bed reactor". Chemical Engineering and Processing. 35 (4): 311–317. doi:10.1016/0255-2701(95)04142-7.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Morelock, C. R. (1961). "Research Laboratory Report #61-RL-2672M" (Document). General Electric.
- ^ Aziz, M. J.; Nygren, E.; Hays, J. F.; Turnbull, D. (1985). "Crystal Growth Kinetics of Boron Oxide Under Pressure". Journal of Applied Physics. 57 (6): 2233. doi:10.1063/1.334368.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Gurr, G. E.; Montgomery, P. W.; Knutson, C. D.; Gorres, B. T. (1970). "The crystal structure of trigonal diboron trioxide". Acta Crystallographica B. 26 (7): 906–915. doi:10.1107/S0567740870003369.
- ^ Strong, S. L.; Wells, A. F.; Kaplow, R. (1971). "On the crystal structure of B2O3". Acta Crystallographica B. 27 (8): 1662–1663. doi:10.1107/S0567740871004515.
- ^ Effenberger, H.; Lengauer, C. L.; Parthé, E. (2001). "Trigonal B2O3 with Higher Space-Group Symmetry: Results of a Reevaluation". Monatshefte für Chemie. 132 (12): 1515–1517. doi:10.1007/s007060170008.
- ^ Mukhanov, V. A.; Kurakevich, O. O.; Solozhenko, V. L. (2008). "On the Hardness of Boron (III) Oxide". Journal of Superhard Materials. 30: 71. doi:10.3103/S1063457608010097.
{{cite journal}}: CS1 maint: multiple names: authors list (link)

