Chalcone

| |

| |
| Names | |
|---|---|
| Preferred IUPAC name
Chalcone | |
| Systematic IUPAC name
(2E)-1,3-Diphenylprop-2-en-1-one | |
| Other names
Chalkone
Benzylideneacetophenone Phenyl styryl ketone | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.002.119 |
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C15H12O | |
| Molar mass | 208.260 g·mol−1 |
| Density | 1.071 g/cm3 |
| Melting point | 55 to 57 °C (131 to 135 °F; 328 to 330 K) |
| Boiling point | 345 to 348 °C (653 to 658 °F; 618 to 621 K) |
| -125.7·10−6 cm3/mol | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Chalcone is an aromatic ketone and an enone that forms the central core for a variety of important biological compounds, which are known collectively as chalcones or chalconoids. Alternative names for chalcone include benzylideneacetophenone, phenyl styryl ketone, benzalacetophenone, β-phenylacrylophenone, γ-oxo-α,γ-diphenyl-α-propylene, and α-phenyl-β-benzoylethylene.
Chemical properties
Chalcones have two absorption maximums at 280 nm and 340 nm.[2]
Chemical reactions
Synthesis
Chalcones can be prepared by an aldol condensation between benzaldehyde and acetophenone in the presence of sodium hydroxide as a catalyst.
This reaction can be carried out without any solvent as a solid-state reaction.[3] The reaction between substituted benzaldehydes and acetophenones can be used as an example of green chemistry in undergraduate education.[4] In a study investigating green syntheses, chalcones were synthesized from the same starting materials in high-temperature water (200 to 350 °C).[5]
Substituted chalcones were also synthesised by piperidine-mediated condensation to avoid side reactions such as multiple condensations, polymerizations, and rearrangements.[6]
Other reactions
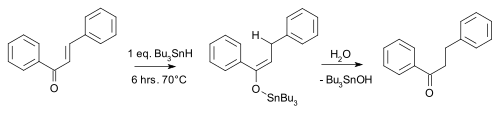
An example is the conjugate reduction of the enone by tributyltin hydride:[7]
3,5-Disubstituted 1H-pyrazoles can be produced from a suitably substituted chalcone by reaction with hydrazine hydrate in the presence of elemental sulfur[8] or sodium persulfate,[9] or by using a hydrazone in which case an azine is produced as a by-product. The specific case for formation of 3,5-diphenyl-1H-pyrazole from chalcone itself can be represented as:[10]
Potential pharmacology
Chalcones and their derivatives demonstrate a wide range of biological activities including anti-inflammation.[11] Some 2′-amino chalcones are have been studied as potential antitumor agents.[12][13]
See also
References
- ^ Merck Index, 11th Edition, 2028
- ^ Song, Dong-mee; Jung, Kyoung-Hoon; Moon, Ji-hye; Shin, Dong-Myung (2003). "Photochemistry of chalcone and the application of chalcone-derivatives in photo-alignment layer of liquid crystal display". Optical Materials. 21: 667–71. Bibcode:2003OptMa..21..667S. doi:10.1016/S0925-3467(02)00220-3.
- ^ Toda, Fumio; Tanaka, Koichi; Hamai, Koki (1990). "Aldol condensations in the absence of solvent: Acceleration of the reaction and enhancement of the stereoselectivity". Journal of the Chemical Society, Perkin Transactions 1 (11): 3207–9. doi:10.1039/P19900003207.
- ^ Palleros, Daniel R (2004). "Solvent-Free Synthesis of Chalcones". Journal of Chemical Education. 81 (9): 1345. Bibcode:2004JChEd..81.1345P. doi:10.1021/ed081p1345.
- ^ Comisar, Craig M; Savage, Phillip E (2004). "Kinetics of crossed aldol condensations in high-temperature water". Green Chemistry. 6 (4): 227–31. doi:10.1039/b314622g.
- ^ Venkatesan, P; Sumathi, S (2009). "Piperidine mediated synthesis ofn-heterocyclic chalcones and their antibacterial activity". Journal of Heterocyclic Chemistry. 47 (1): 81–84. doi:10.1002/jhet.268.
- ^ Leusink, A.J; Noltes, J.G (1966). "Reaction of organotin hydrides with α,β-unsaturated ketones". Tetrahedron Letters. 7 (20): 2221–5. doi:10.1016/S0040-4039(00)72405-1.
- ^ Outirite, Moha; Lebrini, Mounim; Lagrenée, Michel; Bentiss, Fouad (2008). "New one step synthesis of 3,5-disubstituted pyrazoles under microwave irradiation and classical heating". Journal of Heterocyclic Chemistry. 45 (2): 503–5. doi:10.1002/jhet.5570450231.
- ^ Zhang, Ze; Tan, Ya-Jun; Wang, Chun-Shan; Wu, Hao-Hao (2014). "One-Pot Synthesis of 3,5-Diphenyl-1H-pyrazoles from Chalcones and Hydrazine under Mechanochemical Ball Milling". Heterocycles. 89: 103–12. doi:10.3987/COM-13-12867.
- ^ Lasri, Jamal; Ismail, Ali I. (2018). "Metal-free and FeCl3-catalyzed synthesis of azines and 3,5-diphenyl-1H-pyrazole from hydrazones and/or ketones monitored by high resolution ESI+-MS". Indian Journal of Chemistry - Section B. 57B (3): 362–373.
- ^ Mahapatra, Debarshi Kar; Bharti, Sanjay Kumar; Asati, Vivek (2017). "Chalcone Derivatives: Anti-inflammatory Potential and Molecular Targets Perspectives". Current Topics in Medicinal Chemistry. 17 (28): 3146–3169. doi:10.2174/1568026617666170914160446. PMID 28914193.
- ^ Xia, Yi; Yang, Zheng-Yu; Xia, Peng; Bastow, Kenneth F.; Nakanishi, Yuka; Lee, Kuo-Hsiung (2000). "Antitumor agents. Part 202: Novel 2′-amino chalcones: design, synthesis and biological evaluation". Bioorganic & Medicinal Chemistry Letters. 10 (8): 699–701. doi:10.1016/S0960-894X(00)00072-X. ISSN 0960-894X.
- ^ Santos, Mariana B.; Pinhanelli, Vitor C.; Garcia, Mayara A.R.; Silva, Gabriel; Baek, Seung J.; França, Suzelei C.; Fachin, Ana L.; Marins, Mozart; Regasini, Luis O. (2017). "Antiproliferative and pro-apoptotic activities of 2′- and 4′-aminochalcones against tumor canine cells". European Journal of Medicinal Chemistry. 138: 884–889. doi:10.1016/j.ejmech.2017.06.049. ISSN 0223-5234.