From Wikipedia, the free encyclopedia
Dactylifric acid
Names
IUPAC name
5-{[(2Z)-3-(3,4-dihydroxyphenyl)prop-2-enoyl]oxy}-3,4-dihydroxycyclohex-1-ene-1-carboxylic acid
Identifiers
ChemSpider
InChI=1S/C16H16O8/c17-10-3-1-8(5-11(10)18)2-4-14(20)24-13-7-9(16(22)23)6-12(19)15(13)21/h1-5,7,12-13,15,17-19,21H,6H2,(H,22,23)/b4-2+/t12-,13-,15-/m1/s1
Key: MRDAXWGGWWDUKL-GDDAOPKQSA-N
InChI=1/C16H16O8/c17-10-3-1-8(5-11(10)18)2-4-14(20)24-13-7-9(16(22)23)6-12(19)15(13)21/h1-5,7,12-13,15,17-19,21H,6H2,(H,22,23)/b4-2+/t12-,13-,15-/m1/s1
Key: MRDAXWGGWWDUKL-GDDAOPKQBO
C1[C@H]([C@H]([C@@H](C=C1C(=O)O)OC(=O)/C=C/C2=CC(=C(C=C2)O)O)O)O
Properties
C16 H16 O8
Molar mass
336.29 g/mol
Except where otherwise noted, data are given for materials in their
standard state (at 25 °C [77 °F], 100 kPa).
Chemical compound
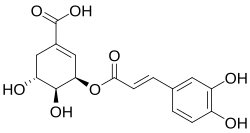
Dactylifric acid is a caffeic acid glycoside (3-O-caffeoylshikimic acid ). It and its isomers are enzymic browning substrates found in dates (Phoenix dactylifera [1]
References
^ Maier, VP; Metzler, DM; Huber, AF (1964). "3-O-Caffeoylshikimic acid (dactylifric acid) and its isomers, a new class of enzymic browning substrates". Biochemical and biophysical research communications . 14 : 124–8. PMID 5836492 .
External links
Aglycones
Precursor Monohydroxycinnamic acids Dihydroxycinnamic acids Trihydroxycinnamic acids O -methylated formsothers
Esters
glycoside-likes
Esters of
Glycosides
Tartaric acid estersOther esters Caffeoyl phenylethanoid
Echinacoside Calceolarioside A , B , C , F Chiritoside A , B , C Cistanoside A , B , C , D , E , F , G , H Conandroside Myconoside Pauoifloside Plantainoside A Plantamajoside Tubuloside B Verbascoside (Isoverbascoside , 2′-Acetylverbascoside )
Oligomeric forms
Dimers
Diferulic acids (DiFA) : 5,5′-Diferulic acid , 8-O -4′-Diferulic acid , 8,5′-Diferulic acid , 8,5′-DiFA (DC) , 8,5′-DiFA (BF) , 8,8′-Diferulic acid Trimers Tetramers
Conjugates withcoenzyme A (CoA)
Template:Natural phenol-stub