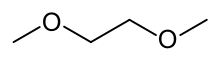
Dimethoxyethane
| |

| |
| Names | |
|---|---|
| Preferred IUPAC name
1,2-Dimethoxyethane[1] | |
| Other names
Ethane-1,2-diyl dimethyl ether[1]
DME Glyme Ethylene glycol dimethyl ether Monoglyme Dimethyl glycol Dimethyl cellosolve | |
| Identifiers | |
3D model (JSmol)
|
|
| Abbreviations | dme |
| 1209237 | |
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.003.451 |
| EC Number |
|
| 1801 | |
PubChem CID
|
|
| RTECS number |
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C4H10O2 | |
| Molar mass | 90.122 g·mol−1 |
| Appearance | Colorless liquid |
| Density | 0.8683 g/cm3 |
| Melting point | −58 °C (−72 °F; 215 K) |
| Boiling point | 85 °C (185 °F; 358 K) |
| miscible | |
| Hazards | |
| GHS labelling: | |
  
| |
| Danger | |
| H225, H332, H360FD | |
| P201, P202, P210, P233, P240, P241, P242, P243, P261, P271, P280, P281, P303+P361+P353, P304+P312, P304+P340, P308+P313, P312, P370+P378, P403+P235, P405, P501 | |
| NFPA 704 (fire diamond) | |
| Flash point | −2 °C (28 °F; 271 K) |
| Related compounds | |
Related Ethers
|
Dimethoxymethane |
Related compounds
|
Ethylene glycol 1,4-Dioxane Diethylene glycol dimethyl ether |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Dimethoxyethane, also known as glyme, monoglyme, dimethyl glycol, ethylene glycol dimethyl ether, dimethyl cellosolve, and DME, is a colorless, aprotic, and liquid ether that is used as a solvent, especially in batteries.[2] Dimethoxyethane is miscible with water.
Production
Monoglyme is produced industrially by the reaction of dimethylether with ethylene oxide:[3][4]
- CH3OCH3 + CH2CH2O → CH3OCH2CH2OCH3
Applications as solvent and ligand

Together with a high-permittivity solvent (e.g. propylene carbonate), dimethoxyethane is used as the low-viscosity component of the solvent for electrolytes of lithium batteries. In the laboratory, DME is used as a coordinating solvent.
Dimethoxyethane is often used as a higher boiling alternative to diethyl ether and THF. Dimethoxyethane acts as a bidentate ligand for some metal cations. It is therefore often used in organometallic chemistry. Grignard reactions and hydride reductions are typical application. It is also suitable for palladium-catalyzed reactions including Suzuki reactions and Stille couplings. Dimethoxyethane is also a good solvent for oligo- and polysaccharides.
References
- ^ a b Nomenclature of Organic Chemistry : IUPAC Recommendations and Preferred Names 2013 (Blue Book). Cambridge: The Royal Society of Chemistry. 2014. p. 704. doi:10.1039/9781849733069-00648. ISBN 978-0-85404-182-4.
- ^ D. Berndt, D. Spahrbier, "Batteries" in Ullmann’s Encyclopedia of Industrial Chemistry 2005, Wiley-VCH, Weinheim. doi:10.1002/14356007.a03_343
- ^ "Ethylene Glycol". Ullmann’s Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. 2000. doi:10.1002/14356007.a10_101. ISBN 3527306730.
{{cite encyclopedia}}: Unknown parameter|authors=ignored (help) - ^ Dimethoxyethane
- ^ Arteaga-Müller, Rocío; Tsurugi, Hayato; Saito, Teruhiko; Yanagawa, Masao; Oda, Seiji; Mashima, Kazushi (2009). "New Tantalum Ligand-Free Catalyst System for Highly Selective Trimerization of Ethylene Affording 1-Hexene: New Evidence of a Metallacycle Mechanism". Journal of the American Chemical Society. 131 (15): 5370–5371. doi:10.1021/ja8100837. PMID 20560633.

