Disilene
| |
| Identifiers | |
|---|---|
| ChemSpider | |
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| Properties | |
| Si 2H 4 | |
| Molar mass | 60.2028 g mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
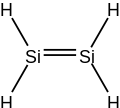
Disilene /daɪsaɪliːn/ (systematically named disilicon tetrahydride) is an inorganic compound with the chemical formula Si
2H
4. The name disilene, referring to the structure of a particular prototropic tautomer of the molecule. It is the simplest silene.
Properties and bonding
Disilene is a molecule with one Si=Si bond, and four equivalent Si-H bonds.
Unlike ethylene, disilene is kinetically unstable with respect to tautomerisation. Disilene has two other tautomers, that are very close in energy: (μ2-H)disilene, and disilanylidene.[1]
Organodisilenes

Disilenes bearing sterically bulky substituents are isolable and have been well characterized although they remain mainly of academic interest. The first stabilised disilene was tetramesityldisilene, (C6Me3H2)4Si2. The Si=Si distance in this molecule is 2.15 Å, about 10% shorter than a typical Si–Si single bond. The Si2C4 core is roughly planar.[2] Such species are typically prepared by reduction of organosilicon halides: 2 R2SiCl2 + 4 Na → R2Si=SiR2 + 4 NaCl An alternative synthesis involves photolysis of trisilacyclopropanes. When the R group is not bulky, cyclic or polymeric polysilanes are the products.
In one study [3] a disilene is prepared by an intramolecular coupling of a 1,1-dibromosilane with potassium graphite. The silicon double bond in the resulting compound has a bond length of 227 picometer (second largest ever found) with trans-bent angles 33° and 31° (by X-ray diffraction).
In addition to this the substituents around the Si-Si bond are twisted by 43°. The disilene isomerizes to a tetracyclic compound by heating at 110°C in xylene thereby releasing its strain energy[clarification needed].
References
- ^ McCarthy, M. C.; Yu, Z.; Sari, L.; Schaefer, H. F.; Thaddeus, P. (15 February 2006). "Monobridged Si2H4". The Journal of Chemical Physics. 124 (7). USA: American Institute of Physics. doi:10.1063/1.2168150.
- ^ Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 978-0-08-037941-8.
- ^ Fused Tricyclic Disilenes with Highly Strained Si-Si Double Bonds: Addition of a Si-Si Single Bond to a Si-Si Double Bond Ryoji Tanaka, Takeaki Iwamoto, and Mitsuo Kira Angewandte Chemie International Edition Volume 45, Issue 38 , Pages 6371 - 6373 2006 doi:10.1002/anie.200602214

