Herniarin
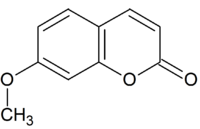
| |
| Names | |
|---|---|
| IUPAC name
7-Methoxychromen-2-one
| |
| Other names
7-O-Methylumbelliferone
7-Methoxycoumarin Ayapanin Herniarine Methyl umbelliferyl ether | |
| Identifiers | |
| |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.007.741 |
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C10H8O3 | |
| Molar mass | 176.171 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Herniarin is a natural chemical compound. Chemically, it can be considered a methoxy derivative of coumarin or a methyl derivative of umbelliferone.
Herniarin is found in Herniaria glabra,[1] Ayapana triplinervis and in species of the genus Prunus (P. mahaleb, P. pensylvanica, and P. maximowiczii).[2]
References
- ^ "Herniarin". liberherbarum.com.
- ^ Santamour F. S. and Riedel L. G. H. (1994). "Distribution and inheritance of scopolin and herniarin in some Prunus species". Biochemical systematics and ecology. 22 (2): 197–201.
