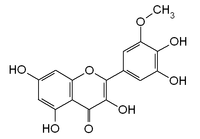
Laricitrin
Appearance
| |
| Names | |
|---|---|
| Other names
3'-O-Methylmyricetin
| |
| Identifiers | |
3D model (JSmol)
|
|
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| Properties | |
| C16H12O8 | |
| Molar mass | 332.26 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Laricitrin is a O-methylated flavonol, a type of flavonoid. It is found in red grape (absent in white grape)[1] and in Vaccinium uliginosum (bog billberries).[2] It is one of the phenolic compounds present in wine.[3]
Metabolism
Laricitrin is formed from myricetin by the action of the enzyme myricetin O-methyltransferase.[4] It is further methylated by laricitrin 5'-O-methyltransferase into syringetin.
Glycosides
- Laricitrin 3-O-galactoside, found in grape[1]
- Laricitrin 3-glucoside found in Larix sibirica[5]
- Laricitrin 3,5’-di-O-β-glucopyranoside, found in Medicago littoralis[6]
References
- ^ a b Metabolite Profiling of Grape: Flavonols and Anthocyanins. Fulvio Mattivi, Raffaele Guzzon, Urska Vrhovsek, Marco Stefanini and Riccardo Velasco, J. Agric. Food Chem., 2006, 54 (20), pp 7692–7702
- ^ Anthocyanin and Flavonol Variation in Bog Bilberries (Vaccinium uliginosum L.) in Finland. Anja K. Lätti, Laura Jaakola, Kaisu R. Riihinen and Pirjo S. Kainulainen, J. Agric. Food Chem., 2010, 58 (1), pp 427–433
- ^ Flavonol profiles of Vitis vinifera red grapes and their single-cultivar wines. Castillo-Munoz Noelia, Gomez-Alonso Sergio, Garcia-Romero Esteban and Hermosin-Gutierrez Isidro, Journal of agricultural and food chemistry, 2007, vol. 55, no3, pp. 992-1002
- ^ Syringetin biosynthetis pathway on metacyc.org
- ^ New flavonol glycosides from the needles of Larix sibirica . N. A. Tyukavkina, S. A. Medvedeva and S. Z. Ivanova, Chemistry of Natural Compounds, Volume 10, Number 2 / march 1974
- ^ Flavonoids isolated from Medicago littoralis Rhode (Fabaceae): their ecological and chemosystematic significance
