Iodomethane

| |||
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
Iodomethane
| |||
| Other names
Methyl iodide, Monoiodomethane, Methyl iodine, MeI, Halon 10001, UN 2644
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ECHA InfoCard | 100.000.745 | ||
| EC Number |
| ||
PubChem CID
|
|||
| RTECS number |
| ||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| CH3I | |||
| Molar mass | 141.94 g/mol | ||
| Appearance | Clear colourless liquid with acrid odor | ||
| Density | 2.2789 g/cm3 at 20 °C | ||
| Melting point | -66.45 °C (206.70 K) | ||
| Boiling point | 42.43 °C (315.58 K) | ||
| 14 g/l at 20 °C | |||
| log P | 1.51 | ||
| Vapor pressure | 50 kPa at 20 °C
53.32 at 25.3 °C 166.1 kPa at 55 °C | ||
| Hazards | |||
| NFPA 704 (fire diamond) | |||
| Flash point | -28 °C | ||
| Explosive limits | 8.5 - 66% | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Iodomethane, commonly called methyl iodide and commonly abbreviated "MeI", is the chemical compound with the formula CH3I. This dense volatile liquid is related to methane by replacement of one hydrogen atom by an atom of iodine and its dipole moment is 1.59 D. Refractive index is 1.5304 (20 °C, D), 1.5293 (21 °C, D). It is miscible with common organic solvents. It is colourless, although upon exposure to light, samples develop a purplish tinge caused by the presence of I2. Storage over copper metal absorbs the iodine. Methyl iodide is widely used in organic synthesis to deliver a methyl group, via the transformation called methylation. It is naturally emitted by rice plantations in small amounts.[1]
Chemical properties
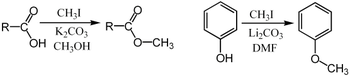
Methyl iodide is an excellent substrate for SN2 substitution reactions. It is sterically open for attack by nucleophiles, and iodide is a good leaving group. For example, it can be used for the methylation of phenols or carboxylic acids:[2]
In these examples, the base (K2CO3 or Li2CO3) removes the acidic proton to form the carboxylate or phenoxide anion, which serves as the nucleophile in the SN2 substitution.
Iodide is a "soft" anion which means that methylation with MeI tends to occur at the "softer" end of an ambidentate nucleophile. For example, reaction with thiocyanate ion favours attack at Template:Sulfur rather than "hard" Template:Nitrogen, leading mainly to methyl thiocyanate (CH3SCN) rather than CH3NCS. This behavior is relevant to the methylation of stabilized enolates such as those derived from 1,3-dicarbonyl compounds. Methylation of these and related enolates can occur on the harder oxygen atom or the (usually desired) carbon atom. With methyl iodide, C-alkylation nearly always predominates.
MeI is also an important precursor to methylmagnesium iodide or "MeMgI", which is a common reagent. Because MeMgI forms readily, it is often prepared in instructional laboratories as an illustration of Grignard reagents. The use of MeMgI has been somewhat superseded by the commercially available methyl lithium.
In the Monsanto process, MeI forms in situ from the reaction of methanol and hydrogen iodide. The CH3I then reacts with carbon monoxide in the presence of a rhodium complex to form acetyl iodide, the precursor to acetic acid after hydrolysis. Most acetic acid is prepared by this method.
MeI hydrolyzes at 270 °C forming hydrogen iodide, carbon monoxide and carbon dioxide.
Preparation
Iodomethane is formed via the exothermic reaction that occurs when iodine is added to a mixture of methanol with red phosphorus.[3] The iodinating reagent is phosphorus triiodide that is formed in situ:
Alternatively, it is prepared from the reaction of dimethyl sulfate with potassium iodide in the presence of calcium carbonate:[3]
- (CH3O)2SO2 + KI → K2SO4 + 2 CH3I
The CH3I can be purified by distillation followed by washing with Na2S2O3 to remove iodine.
Methyl iodide forms during nuclear accidents by the reaction of organic matter with the "fission iodine."
Choice of iodomethane as a methylating agent
Iodomethane is an excellent reagent for methylation, but there are some disadvantages to its use. It has a high equivalent weight: one mole of MeI weighs almost three times as much as one mole of methyl chloride. However, the chloride is a gas (as is methyl bromide), making it more awkward to work with than liquid MeI. Methyl chloride is a poorer methylating reagent than MeI, though it is often adequate.
Iodides are generally expensive relative to the more common chlorides and bromides, though iodomethane is reasonably affordable; on a commercial scale the toxic dimethyl sulfate is preferred, since it is both cheap and liquid. The iodide leaving group in MeI may cause side reactions, as it is a powerful nucleophile. Finally, being highly reactive, MeI is more dangerous for laboratory workers than related chlorides and bromides. When considering alternatives to MeI, it is necessary to consider cost, handling, risk, chemical selectivity, and ease of reaction work-up.
Uses
Besides use as a methylation agent, there have been proposals of its use as a fungicide, herbicide, insecticide or nematicide and as a fire extinguisher. Further it can be used as a soil disinfectant, replacing bromomethane (which was banned under the Montreal Protocol), and in microscopy due to properties related to refraction index. In a controversial October 2007 decision, the United States Environmental Protection Agency approved its use as a soil fumigant in some cases, although it cannot yet be used in California (a major potential market) due to lack of state approval.[4]
Toxicity and Biological effects
Iodomethane has an LD50 for oral administration to rats 76 mg/kg, and in the liver it undergoes rapid conversion to S-methylglutathione.[5] Iodomethane is a possible carcinogen based on its IARC, ACGIH, NTP, or EPA classification. According to IARC it is classified as a group 3 substance (Group 3:The agent is not classifiable as to its carcinogenicity to humans).
Breathing iodomethane fumes can cause lung, liver, kidney and central nervous system damage. It causes nausea, dizziness, coughing and vomiting. Prolonged contact with skin causes burns. Massive inhalation causes pulmonary edema.
References
- ^ K. R. Redeker, N.-Y. Wang, J. C. Low, A. McMillan, S. C. Tyler, and R. J. Cicerone (2000). "Emissions of Methyl Halides and Methane from Rice Paddies". Science. 290: 966–969. doi:10.1126/science.290.5493.966. PMID 11062125.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Avila-Zárraga, J. G., Martínez, R. (2001). "Efficient methylation of carboxylic acids with potassium hydroxide/methyl sulfoxide and iodomethane". Synthetic Communications. 31 (14): 2177–2183. doi:10.1081/SCC-100104469.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ a b King, C. S.; Hartman, W. W. (1943). "Methyl Iodide". Organic Syntheses
{{cite journal}}: CS1 maint: multiple names: authors list (link); Collected Volumes, vol. 2, p. 399. - ^ "EPA approves new pesticide despite scientists' concerns". Los Angeles Times. October 6, 2007.
{{cite news}}: Check date values in:|date=(help) - ^ Johnson, M. K. (1966). "Metabolism of iodomethane in the rat". Biochem. J. 98: 38–43.
- March, Jerry (1992), Advanced Organic Chemistry: Reactions, Mechanisms, and Structure (4th ed.), New York: Wiley, ISBN 0-471-60180-2
- Sulikowski, G. A.; Sulikowski, M. M. (1999). in Coates, R.M.; Denmark, S. E. (Eds.) Handbook of Reagents for Organic Synthesis, Volume 1: Reagents, Auxiliaries and Catalysts for C-C Bond Formation New York: Wiley, pp. 423–26.
- Bolt H. M., Gansewendt B. (1993). "Mechanisms of carcinogenicity of methyl halides". Crit Rev Toxicol. 23 (3): 237–53. doi:10.3109/10408449309105011. PMID 8260067.
External links
- International Chemical Safety Card 0509
- NIOSH Pocket Guide to Chemical Hazards. "#0420". National Institute for Occupational Safety and Health (NIOSH).
- IARC Summaries & Evaluations: Vol. 15 (1977), Vol. 41 (1986), Vol. 71 (1999)
- Metabolism of iodomethane in the rat
- Iodomethane NMR spectra