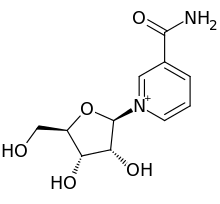
Nicotinamide riboside
| |

| |
| Names | |
|---|---|
| IUPAC name
3-Carbamoyl-1-(β-D-ribofuranosyl)pyridin-1-ium
| |
| Systematic IUPAC name
3-Carbamoyl-1-[(2R,3R,4S,5R)-3,4-dihydroxy-5-(hydroxymethyl)oxolan-2-yl]pyridin-1-ium | |
| Other names
1-(β-D-Ribofuranosyl)nicotinamide; N-Ribosylnicotinamide
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C11H15N2O5+ | |
| Molar mass | 255.25 g/mol |
| Melting point | Complete thermal degradation occurs above 130°C (chloride salt)[1] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Nicotinamide riboside (NR, SR647) is a pyridine-nucleoside and a form of vitamin B3. It functions as a precursor to nicotinamide adenine dinucleotide, or NAD+,[2] through a two-step and a three-step pathway.[3]
Chemistry
[edit]While the molecular weight of nicotinamide riboside is 255.25 g/mol,[4] that of its chloride salt is 290.70 g/mol.[5][6] As such, 100 mg of nicotinamide riboside chloride provides 88 mg of nicotinamide riboside.[citation needed]
Stability and degradation
[edit]NRCl is susceptible to degradation through both thermal decomposition and base-catalyzed hydrolysis:
- Thermal degradation: Decomposition occurs rapidly at temperatures above 130°C, producing nicotinamide (NAM) and ribose.[1]
- Degradation kinetics: NRCl degradation follows pseudo-first-order kinetics, with the degradation rate doubling for every 10°C increase in temperature. At pH 7.4, degradation is significantly faster than in acidic conditions, highlighting the importance of pH control for formulation stability.[1]
These properties emphasize the need for careful formulation strategies to ensure NRCl's stability during storage, processing, and delivery.[citation needed]
History
[edit]Nicotinamide riboside (NR) has been identified as an NAD precursor, involved in salvage NAD synthesis in both bacteria and eukaryotes.[7] In bacteria, it was first described in 1944 as a necessary growth factor for the culture of Haemophilus influenza, H. influenza was identified as requiring both X factor (hemin) and V factor (NAD) to grow.[8] V factor, purified from blood, was shown to exist in three forms: Nicotinamide adenine dinucleotide (NAD+), NMN and NR. NR was the compound that led to the most rapid growth of the H. influenza bacterium.[9][7]
H. influenza cannot grow on nicotinic acid (NA), nicotinamide (NAM), or amino acids such as tryptophan (Trp) or aspartic acid (Asp), which were the previously known precursors of NAD+.[7][10] H. influenza depends entirely on salvage of NAD precursors from other cells in its environment.[9]
The identification of Nicotinamide riboside (NR) as an NAD precursor in eukaryotes developed out of the study of pellagra.[11] Pellagra was the first disease to be associated with NAD+ deficiency.[12] It was linked to nutritional deficiency by Joseph Goldberger in 1914, and to deficiency of niacin (vitamin B3) by Conrad Elvehjem in 1937. NAD+ (then called coenzyme I) was shown to be extremely low in cases of pellagra, and NA and NAM were identified as molecular precursors in rebuilding NAD+ levels. Pellagra is now understood as a severe, chronic depletion of NAD+, which can be treated through diet.[11]
Subsequent studies of NAD+ metabolism have identified regulatory pathways used by cells and tissues to maintain NAD+ availability. NAD+ and its precursors nicotinic acid (NA) and nicotinamide (NAM) have been shown to be vital cofactors in cellular oxidation/reduction reactions and ATP synthesis. Classic NAD+ synthesis pathways characterized in eukaryotes include an eight-step de novo pathway from Trp and two pathways using the NAD+ precursors NA and NAM: a three-step NA-based pathway known as the Preiss-Handler pathway; and an NAM-based pathway involving the enzyme Nicotinamide phosphoribosyltransferase (NAMPT) and the formation of nicotinamide mononucleotide (NMN).[11][13][14]
In 2004, a previously unknown pathway was reported when nicotinamide riboside (NR) was identified as an additional NAD+ precursor in eukaryotes.[11][13][14] NR is now recognized as a form of vitamin B3[15] which can be found in both cow and human milk.[11][16] Once internalized into a cell, NR is rapidly phosphorylated by the activity of nicotinamide riboside kinase enzymes (NRK1 and NRK2) to form nicotinamide mononucleotide (NMN), bypassing the previously known biosynthetic routes to NAD+ production. NMN is then converted to NAD+ by NMN-adenylyltransferase (NMNAT).[13]
Research in mammals indicates that NRK1 is a cytosolic protein, encoded by the Nmrk1 gene. It is found in most tissues but predominantly in the liver and kidney. The NRK2 protein may be related to muscle tissue including cardiac muscle. It is encoded by the Nmrk2 gene and appears to be more highly expressed in cases of metabolic stress or cellular damage.[11][13][14] Since different types of tissues display differing concentrations of NR and NRKs, it is likely that NR utilization will vary in different tissues.[3][13]
Metabolic studies indicate that NAD+, once considered a stable molecule, is continuously turned over and used, requiring tight regulation to maintain metabolic homeostasis. NR utilization in mammals may involve both exogenous dietary sources and endogenous salvage processes that recycle intermediates. NR metabolism and the interactions of different NAD+ pathways continue to be studied. The NAM and NR pathways involve an amide group and are referred to as 'amidated' pathways. The pathways for de novo synthesis from tryptophan and from NA salvage are 'deamidated' pathways, which share a rate-limiting amidation enzyme NADsynthase1 (NADSYN).[13][11] Disruptions or imbalances in NAD+ metabolism have been observed in many disease conditions, and the possibility of restoring NAD+ levels by administering NAD+ precursors is an area of interest for researchers.[11][12][13]
Biosynthesis
[edit]Nicotinamide riboside (NR) is now known to be an NAD+ precursor, involved in the biosynthetic pathways that convert B3 vitamins into NAD+. NAD+ is primarily synthesized in mammals de novo from tryptophan, through the Priess-Handler pathway from nicotinic acid (NA) or via a salvage pathway from nicotinamide (NAM).[17]

Nicotinamide riboside (NR) is utilized through an additional pathway involving phosphorylation by the nicotinamide riboside kinase enzymes (NRK1 and NRK2).[17][13] In yeasts, NR has also been shown to be degraded by the nucleosidases Pnp1, Urh1 and Meu1, before being converted to NAD⁺ via the Preiss-Handler pathway and the action of the nicotinamidase Pnc1.[3][10]
Commercialization
[edit]ChromaDex licensed patents in July 2012, and began to develop a process to bring NR to market as TruNiagen.[18] ChromaDex has been in a patent dispute with Elysium Health over the rights to nicotinamide riboside supplements since 2016.[19]
Safety designations
[edit]In 2016, the U.S. Food and Drug Administration (FDA) has granted Generally recognized as safe (GRAS) status to ChromaDex for its preparation of nicotinamide riboside chloride (NRC, Niagen™).[5][20][3] It was designated a new dietary ingredient (NDI) for use in dietary supplements by the U.S. Food and Drug Administration in 2015 and 2017. It was listed in Health Canada's Licensed Natural Health Products Database (LNHPD) in 2018. The European Union has granted NRC a "New dietary ingredient" designation as a novel food pursuant to Regulation (EU) 2015/2283, as of 2019. It was authorized for use in food supplements by the EU in 2020. The EFSA Panel on Nutrition, Novel Foods and Food Allergens (NDA) considered it as safe as pure nicotinamide for use in food for special medical purposes (FSMP) and total diet replacement for weight control (TDRWC) in adults as of 2021 but noted that further investigation would be required to establish safety for some other types of use.[21] The Australian government has given nicotinamide riboside chloride a positive listing under the compositional guidelines of its Therapeutic Goods Administration (TGA).[22]
See also
[edit]- Nicotinamide mononucleotide
- Niacin
- Nicotinamide
- Vitamin B3
- Nicotinamide adenine dinucleotide
- Sirtuin
- Poly (ADP-ribose) polymerase
References
[edit]- ^ a b c Campbell, MT; Jones, DS; Andrews, GP; Li, S (2019). "Understanding the physicochemical properties and degradation kinetics of nicotinamide riboside chloride". Food & Nutrition Research. 63: 3419. doi:10.29219/fnr.v63.3419. PMC 6878970. PMID 31807125.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Bogan, KL; Brenner, C (2008). "Nicotinic acid, nicotinamide, and nicotinamide riboside: a molecular evaluation of NAD+ precursor vitamins in human nutrition". Annu. Rev. Nutr. 28: 115–130. doi:10.1146/annurev.nutr.28.061807.155443. PMID 18429699.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b c d Mehmel, M; Jovanović, N; Spitz, U (31 May 2020). "Nicotinamide Riboside-The Current State of Research and Therapeutic Uses". Nutrients. 12 (6): 1616. doi:10.3390/nu12061616. PMC 7352172. PMID 32486488.
- ^ "Nicotinamide riboside". pubchem.ncbi.nlm.nih.gov. Archived from the original on 2017-02-20. Retrieved 2017-02-20.
- ^ a b "GRAS Notices, GRN No. 635". www.accessdata.fda.gov. Archived from the original on 11 February 2017. Retrieved 18 February 2019.
- ^ "Spherix/ChromaDex GRAS submission" (PDF). FDA.gov. Archived (PDF) from the original on 4 March 2017. Retrieved 18 February 2019.
- ^ a b c Gazzaniga, F; et al. (September 2009). "Microbial NAD metabolism: lessons from comparative genomics". Microbiology and Molecular Biology Reviews. 73 (3): 529–41. doi:10.1128/MMBR.00042-08. PMC 2738131. PMID 19721089.
- ^ World Health Organization; Centers for Disease Control and Prevention (2011). "Chapter 9 Identification and Characterization of Haemophilus influenzae". Laboratory Methods for the Diagnosis of Meningitis caused by Neisseria meningitidis, Streptococcus pneumoniae, and Haemophilus influenzae (PDF) (Second ed.). Geneva, Switzerland: WHO Press, World Health Organization. pp. 87–104. Archived (PDF) from the original on 2023-02-12. Retrieved 2023-02-12.
- ^ a b Gingrich, W; Schlenk, F (June 1944). "Codehydrogenase I and Other Pyridinium Compounds as V-Factor for Hemophilus influenzae and H. parainfluenzae". Journal of Bacteriology. 47 (6): 535–50. doi:10.1128/JB.47.6.535-550.1944. PMC 373952. PMID 16560803.
- ^ a b Belenky, P.; et al. (2007). "NAD+ Metabolism in Health and Disease". Trends in Biochemical Sciences. 32 (1): 12–19. doi:10.1016/j.tibs.2006.11.006. PMID 17161604.
- ^ a b c d e f g h Cercillieux, A; Ciarlo, E; Canto, C (2022-08-02). "Balancing NAD+ deficits with nicotinamide riboside: therapeutic possibilities and limitations". Cellular and Molecular Life Sciences. 79 (8): 463. doi:10.1007/s00018-022-04499-5. ISSN 1420-9071. PMC 9345839. PMID 35918544.
- ^ a b Katsyuba, E; Romani, M; Hofer, D; Auwerx, J (January 2020). "NAD(+) homeostasis in health and disease". Nature Metabolism. 2 (1): 9–31. doi:10.1038/s42255-019-0161-5. ISSN 2522-5812. PMID 32694684. S2CID 214277961. Archived from the original on 11 November 2022. Retrieved 6 February 2023.
- ^ a b c d e f g h Fletcher RS, Lavery GG (2018). "The emergence of the nicotinamide riboside kinases in the regulation of NAD+ metabolism". Journal of Molecular Endocrinology. 61 (1): R107–R121. doi:10.1530/JME-18-0085. PMC 6145238. PMID 30307159.
- ^ a b c Reiten, OK; Wilvang, MA; Mitchell, SJ; Hu, Z; Fang, EF (October 2021). "Preclinical and clinical evidence of NAD(+) precursors in health, disease, and ageing". Mechanisms of Ageing and Development. 199: 111567. doi:10.1016/j.mad.2021.111567. hdl:20.500.11850/506217. PMID 34517020. S2CID 237459655. Archived from the original on 2023-02-13. Retrieved 2023-02-13.
- ^ Chi, Y; Sauve, AA (November 2013). "Nicotinamide riboside, a trace nutrient in foods, is a vitamin B3 with effects on energy metabolism and neuroprotection". Current Opinion in Clinical Nutrition and Metabolic Care. 16 (6): 657–61. doi:10.1097/MCO.0b013e32836510c0. PMID 24071780. S2CID 205782942. Archived from the original on 2023-02-13. Retrieved 2023-02-13.
- ^ Ummarino, S; Mozzon, M; Zamporlini, F; Amici, A; Mazzola, F; Orsomando, G; Ruggieri, S; Raffaelli, N (15 April 2017). "Simultaneous quantitation of nicotinamide riboside, nicotinamide mononucleotide and nicotinamide adenine dinucleotide in milk by a novel enzyme-coupled assay". Food Chemistry. 221: 161–168. doi:10.1016/j.foodchem.2016.10.032. PMID 27979136. Archived from the original on 16 February 2023. Retrieved 16 February 2023.
- ^ a b McReynolds, MR; Chellappa, K; Baur, JA (22 February 2020). "Age-related NAD(+) decline". Experimental Gerontology. 134: 110888. doi:10.1016/j.exger.2020.110888. PMC 7442590. PMID 32097708.
- ^ "ChromaDex Licenses Exclusive Patent Rights for Nicotinamide Riboside (NR) Vitamin Technologies". 2012-07-16. Archived from the original on 2019-02-15. Retrieved 15 February 2019.
- ^ Bomgardner, Melody M. (2018). "Firms feud over purported age-fighting molecule". Chemical & Engineering News. 96 (33). Archived from the original on 2022-11-16. Retrieved 2022-11-16.
- ^ "GRAS Notice (GRN) No. 635" (PDF). FDA. Archived (PDF) from the original on 2 January 2023. Retrieved 15 February 2023.
- ^ Turck, D; et al. (November 2021). "Extension of use of nicotinamide riboside chloride as a novel food pursuant to Regulation (EU) 2015/2283". EFSA Journal. 19 (11): e06843. doi:10.2903/j.efsa.2021.6843. PMC 8586847. PMID 34804232.
- ^ "Nicotinamide riboside chloride". Department of Health and Aged Care, Commonwealth of Australia. Archived from the original on 16 February 2023. Retrieved 16 February 2023.
