Octaazacubane
| |

| |
| Names | |
|---|---|
| Other names
Octaazapentacyclo[4.2.0.02,5.03,8.04,7]octane; Cubaazane; Nitrogen octaatomic molecule
| |
| Identifiers | |
3D model (JSmol)
|
|
CompTox Dashboard (EPA)
|
|
| |
| Properties | |
| N8 | |
| Molar mass | 112.056 g·mol−1 |
| Density | 2.69 g/cm3 (predicted)[1] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
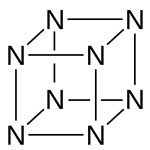
Octaazacubane is a hypothetical allotrope of nitrogen with formula N8, whose molecules have eight atoms arranged into a cube. (By comparison, nitrogen usually occurs as the diatomic molecule N2.) It can be regarded as a derivative of cubane, where all eight carbon atoms (and their corresponding hydrogen atoms) have been replaced with a nitrogen atom.[2] It is predicted to be a metastable molecule, in which despite the thermodynamic instability caused by bond strain, and the high energy of the N-N single bonds, the molecule remains kinetically stable for reasons of orbital symmetry.[3]
Explosive and fuel
Octaazacubane is predicted to have an energy density (assuming decomposition into N2) of 22.9 MJ / kg,[4] which is over 5 times the standard value of TNT. It has therefore been proposed (along with other exotic nitrogen allotropes) as an explosive, and as a component of high performance rocket fuel.[5] Its velocity of detonation is predicted to be 15,000 m/s, much (48.5%) more than ONC, the fastest known nonnuclear explosive.[6]
See also
- Tetranitrogen (Nitrogen allotrope with formula N4)
- Hexazine (Nitrogen allotrope with formula N6)
- Azidopentazole (Nitrogen allotrope with formula N8)
- Bispentazole (Nitrogen allotrope with formula N10)[7]
- Bis(pentazolyl)diazene (Nitrogen allotrope with formula N12)
- Eicosaazadodecahedrane (Nitrogen allotrope with formula N20)[8]
- Hexacontaazabuckminsterfullerene (Nitrogen allotrope with formula N60)[9][10]
- Pentazole
- Octanitrocubane (ONC)
- 1,1'-Azobis-1,2,3-triazole
References
- ^ Agrawal, Jai Prakash (2010). High Energy Materials: Propellants, Explosives and Pyrotechnics. Online: Wiley-VCH. p. 498. ISBN 978-3-527-62880-3.
- ^ B. Muir. "Cubane"(See under "further topics" section.)
{{cite web}}: CS1 maint: postscript (link) - ^ Template:Cite article
- ^ Template:Cite article
- ^ Template:Cite article
- ^ Agrawal, Jai Prakash (2010). High Energy Materials: Propellants, Explosives and Pyrotechnics. Online: Wiley-VCH. p. 498. ISBN 978-3-527-62880-3.
- ^ Manaa, M. R. (2000). "Toward new energy-rich molecular systems: From N10 to N60". Chemical Physics Letters. 331 (2–4): 262–268. doi:10.1016/S0009-2614(00)01164-7.
- ^ Charkin, O. P. (2013). "Theoretical study of N20, C20, and B20 clusters "squeezed" inside icosahedral C80 and He80 cages". Russian Journal of Inorganic Chemistry. 58: 46–55. doi:10.1134/S0036023613010038.
- ^ Wang, L. J.; Zgierski, M. Z. (2003). "Super-high energy-rich nitrogen cluster N60". Chemical Physics Letters. 376 (5–6): 698. doi:10.1016/S0009-2614(03)01058-3.
- ^ https://www.llnl.gov/str/June01/Manaa.html
