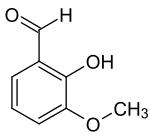
ortho-Vanillin
| |
| Names | |
|---|---|
| IUPAC name
2-Hydroxy-3-methoxybenzaldehyde
| |
| Other names
o-Vanillin
3-Methoxysalicylaldehyde | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.005.197 |
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C8H8O3 | |
| Molar mass | 152.15 g/mol |
| Appearance | Yellow, fibrous solid |
| Density | 1.231 g/mL |
| Melting point | 40 to 42 °C (104 to 108 °F; 313 to 315 K) |
| Boiling point | 265 to 266 °C (509 to 511 °F; 538 to 539 K) |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards
|
May cause irritation to skin, eyes, and respiratory tract |
| Flash point | > 110 °C (230 °F; 383 K) |
| Safety data sheet (SDS) | External MSDS |
| Related compounds | |
Related compounds
|
Eugenol, Anisaldehyde, Phenol, Vanillin |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
ortho-Vanillin (2-Hydroxy-3-methoxybenzaldehyde) is an organic solid present in the extracts and essential oils of many plants.[1][2][3] Its functional groups include aldehyde, ether and phenol. ortho-Vanillin, a compound of the formula C8H8O3, is distinctly different from its more prevalent isomer, vanillin. The "ortho-" prefix refers to the position of the compound’s hydroxyl moiety, which is found in the para-position in vanillin.
ortho-Vanillin is a fibrous, light-yellow, crystalline solid. Present in a variety of food products, it is not specifically sought after, and is therefore a less-commonly produced and encountered food additive.
History
ortho-Vanillin was first isolated, in 1876, by renowned German chemist Ferdinand Tiemann.[4] By 1910, methods for its purification had been developed by Francis Noelting, who similarly demonstrated its versatility as a general synthetic precursor for a diverse array of compounds, such as the coumarins.[5]
By 1920, the compound began to show use as a dye for hides.[6]
Biological properties
ortho-Vanillin is harmful if ingested, irritating to eyes, skin and respiratory system, but has an unmistakable high LD50 of 1330 mg/kg in mice.[7]
It is a weak inhibitor of tyrosinase,[8] and displays both antimutagenic and comutagenic properties in Escherichia coli.[9] However, its net effect makes it a “potent comutagen.”[10]
ortho-Vanillin possesses moderate antifungal and antibacterial properties.[11]
Uses
Today, most ortho-vanillin is used in the study of mutagenesis and as a synthetic precursor for pharmaceuticals, for example, benafentrine[12] and an antiandrogen compound called Pentomone.
See also
Notes
- ^ Abou Zeid, A. H.; Sleem, A. A. (2002). "Natural and stress constituents from Spinacia oleracea L. leaves and their biological activities". Bulletin of the Faculty of Pharmacy (Cairo University). 40 (2): 153–167.
- ^ Barbe, Jean-Christophe; Bertrand, Alain. (1996). "Quantitative analysis of volatile compounds stemming from oak wood. Application to the aging of wines in barrels". Journal des Sciences et Techniques de la Tonnellerie. 2: 77–88.
- ^ Brunke, E. J.; Hammerschmidt, F. J.; Schmaus, G. (1992). "Das etherische Öl von Santolina chamaecyparissus L. (Santolina chamaecyparissus essential oil)". Parfümerie und Kosmetik. 73 (9): 617–618, 623–624, 626, 628–630, 632, 634–637.
- ^ Tiemann, Ferdinand (1876). "Ueber die der Coniferyl- und Vanillinreihe angehörigen Verbindungen (Coniferyl- and vanillin series-related compounds)". Berichte der Deutschen Chemischen Gesellschaft. 9: 409–423. doi:10.1002/cber.187600901133.
- ^ Noelting, Francis A. M. o-Hydroxy-m-methoxybenzaldehyde (Orthovanillin). Annales de Chimie et de Physique (1910), 19, 476–550.
- ^ Gerngross, Otto. Dyeing hide with o-vanillin and o-protocatechualdehyde and the aldehyde tanning. Angewandte Chemie (1920), 33 (44), 136–138.
- ^ http://msds.chem.ox.ac.uk/VA/o-vanillin.html
- ^ Kubo, Isao; Kinst-Hori, Ikuyo. Tyrosinase inhibitory activity of the olive oil flavor compounds. Journal of Agricultural and Food Chemistry (1999), 47 (11), 4574–4578.
- ^ Watanabe, Kazuko; Ohta, Toshihiro; Shirasu, Yasuhiko. Enhancement and inhibition of mutation by o-vanillin in Escherichia coli. Mutation Research, DNA Repair (1989), 218 (2), 105–109.
- ^ Takahashi, Kazuhiko; Sekiguchi, Mutsuo; Kawazoe, Yutaka. A specific inhibition of induction of adaptive response by o-vanillin, a potent comutagen. Biochemical and Biophysical Research Communications (1989), 162 (3), 1376–1381.
- ^ Leifertova, I.; Hejtmankova, N.; Hlava, H.; Kudrnacova, J.; Santavy, F. Antifungal and antibacterial effects of phenolic substances. A study of the relation between the biological activity and the constitution of the investigated compounds. Acta Universitatis Palackianae Olomucensis, Facultatis Medicae (1975), 74, 83–101.
- ^ Press, Jeffery B.; Bandurco, Victor T.; Wong, Elizabeth M.; Hajos, Zoltan G.; Kanojia, Ramesh M.; Mallory, Robert A.; Deegan, Edward G.; Mcnally, James J.; Roberts, Jerry R.; Cotter, Mary Lou; Graden, David W.; Lloyd, John R. (1986). "Synthesis of 5,6-dimethoxyquinazolin-2(1H)-ones". Journal of Heterocyclic Chemistry. 23 (6): 1821–1828. doi:10.1002/jhet.5570230643. ISSN 0022-152X.
