Ozonolysis
Ozonolysis is the cleavage of an alkene or alkyne with ozone to form organic compounds in which the multiple carbon-carbon bond has been replaced by a double bond to oxygen.[1][2][3][4] The outcome of the reaction depends on the type of multiple bond being oxidized and the workup conditions.
Ozonolysis of alkenes
Alkenes can be oxidized with ozone to form alcohols, aldehydes or ketones, or carboxylic acids. In a typical procedure, ozone is bubbled through a solution of the alkene in methanol at –78 °C until the solution takes on a characteristic blue color. This indicates consumption of the alkene. A reagent is then added to convert the intermediate ozonide to a carbonyl derivative. Reductive work-up conditions are far more commonly used than oxidative conditions. The use of triphenylphosphine, thiourea, zinc dust, or dimethyl sulfide produces aldehydes or ketones while the use of sodium borohydride produces alcohols. The use of hydrogen peroxide produces carboxylic acids. Recently, the use of amine N-oxides has been reported to produce aldehydes directly.[5] Other functional groups, such as benzyl ethers, can also be oxidized by ozone. It has been proposed that small amounts of acid may be generated during the reaction from oxidation of the solvent, so pyridine is sometimes used to buffer the reaction. Dichloromethane is often used as a 1:1 cosolvent to facilitate timely cleavage of the ozonide. Azelaic acid and pelargonic acids are produced from ozonolysis of oleic acid on an industrial scale.

A widely used alternative method[1] for the ozonolysis of symmetrical alkenes allows differentially terminated hydrocarbons to be generated:
- with O3/MeOH/CH2Cl2; TsOH; sodium hydrogencarbonate (NaHCO3); dimethyl sulfide (DMS), aldehyde and dimethyl acetal termini
- with O3/MeOH/CH2Cl2; acetic anhydride (Ac2O), triethylamine (Et3N), methyl esters and aldehyde termini
- with O3/MeOH/CH2Cl2; TsOH; Ac2O, Et3N, methyl ester and dimethyl acetal termini.
An example is the ozonolysis of eugenol converting the terminal alkene to an aldehyde:[6]
Reaction mechanism

In the generally accepted mechanism proposed by Rudolf Criegee in 1953,[7][8][9] the alkene and ozone form an intermediate molozonide in a 1,3-dipolar cycloaddition. Next, the molozonide reverts to its corresponding carbonyl oxide (also called the Criegee intermediate or Criegee zwitterion) and aldehyde or ketone in a retro-1,3-dipolar cycloaddition. The oxide and aldehyde or ketone react again in a 1,3-dipolar cycloaddition or produce a stable ozonide intermediate (a trioxolane).
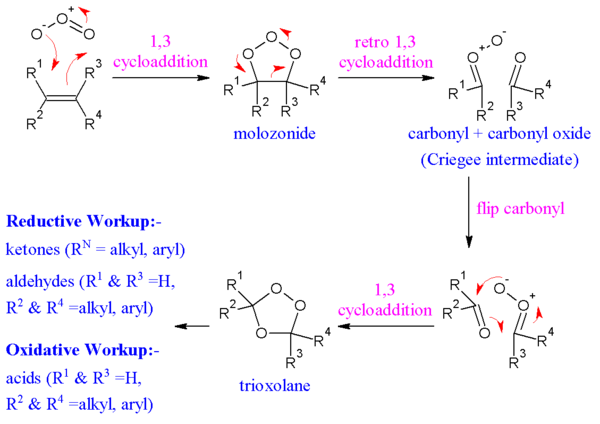
Evidence for this mechanism is found in isotopic labeling. When 17O-labelled benzaldehyde reacts with carbonyl oxides, the label ends up exclusively in the ether linkage of the ozonide.[10] There is still dispute over whether the molozonide collapses via a concerted or radical process; this may also exhibit a substrate dependence.
History
Ozonolysis was invented by Christian Friedrich Schönbein in 1840. Before the advent of modern spectroscopic techniques, it was an important method for determining the structure of organic molecules. Chemists would ozonize an unknown alkene to yield smaller and more readily identifiable fragments. The ozonolysis of alkenes is sometimes referred to as "Harries Ozonolysis," because some attribute this reaction to Carl Dietrich Harries.[11]
Ozonolysis of alkynes
Ozonolysis of alkynes generally gives an acid anhydride or diketone product,[12] not complete fragmentation as for alkenes. A reducing agent is not needed for these reactions. The exact mechanism is not completely known.[13] If the reaction is performed in the presence of water, the anhydride hydrolyzes to give two carboxylic acids.

Ozonolysis of elastomers
The method was used to confirm the structural repeat unit in natural rubber as isoprene. It is also a serious problem, known as "ozone cracking" where traces of the gas in an atmosphere will attack and split double bonds in susceptible elastomers, including natural rubber, polybutadiene, Styrene-butadiene and Nitrile rubber. Ozone cracking creates small cracks at right angles to the load in the surfaces exposed to the gas, the cracks growing steadily as attack continues. The rubber product must be under tension for crack growth to occur.
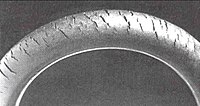
Ozone cracking is a form of stress corrosion cracking where active chemical species attack products of a susceptible material. Ozone cracking is a serious problem in fuel lines since cracks can penetrate the bore and cause a fuel leak, leading to fires or spillage of diesel fuel onto roads for example. Spills are especially hazardous since the slick is extremely slippery and is the cause of many road accidents, especially those involving motorcyclists. Ozone cracking was formerly commonly seen in the tyre sidewalls but is now rare owing to the use of anti-ozonants. Other means of prevention include replacing susceptible rubbers with resistant elastomers such as polychloroprene, EPDM or fluoroelastomers such as Viton.
See also
References
- ^ a b Ronald E. Claus and Stuart L. Schreiber (1990). "Ozonolytic Cleavage of Cyclohexene to Terminally Differentiated Products". Organic Syntheses; Collected Volumes, vol. 7, p. 168.
- ^ Bailey, P. S.; Erickson, R. E. (1973). "Diphenaldehyde". Organic Syntheses
{{cite journal}}: CS1 maint: multiple names: authors list (link); Collected Volumes, vol. 5, p. 489. - ^ Tietze, L. F.; Bratz, M. (1998). "Dialkyl Mesoxalates by Ozonolysis of Dialkyl Benzalmalonates". Organic Syntheses
{{cite journal}}: CS1 maint: multiple names: authors list (link); Collected Volumes, vol. 9, p. 314. - ^ Laurence M. Harwood, Christopher J. Moody (1989-06-13). Experimental organic chemistry: Principles and Practice (Illustrated ed.). WileyBlackwell. pp. 55–57. ISBN 0632020172.
{{cite book}}: Unknown parameter|isbn-13=ignored (help) - ^ Schwartz, Chris; Raible, J; Mott, K; Dussault, PH (2006). "Fragmentation of Carbonyl Oxides by N-Oxides: An Improved Approach to Alkene Ozonolysis". Org. Lett. 8 (15): 3199–3201. doi:10.1021/ol061001k. PMID 16836365.
{{cite journal}}: no-break space character in|title=at position 50 (help) - ^ Bruce M. Branan, Joshua T. Butcher, and Lawrence R. Olsen (2007). "Using Ozone in Organic Chemistry Lab: The Ozonolysis of Eugenol". Journal of Chemical Education. 84: 1979. doi:10.1021/ed084p1979.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Criegee, R. (1975). "Mechanism of Ozonolysis". Angew. Chem. Int. Ed. Engl. 14: 745–752. doi:10.1002/anie.197507451.
- ^ http://www.organic-chemistry.org/namedreactions/ozonolysis-criegee-mechanism.shtm Ozonolysis mechanism on Organic Chemistry Portal site
- ^ Li, Jie Jack: Criegee mechanism of ozonolysis Book: Name Reactions. 2006, 173-174, doi:10.1007/3-540-30031-7_77
- ^ Geletneky, C.; Berger, S. (1998). "The Mechanism of Ozonolysis Revisited by 17O-NMR Spectroscopy". Eur. J. Org. Chem.: 1625–1627.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Mordecai B. Rubin (2003). "The History of Ozone Part III, C. D. Harries and the Introduction of Ozone into Organic Chemistry". Helvetica Chimica Acta. 86 (4): 930–940. doi:10.1002/hlca.200390111.
- ^ Bailey, P. S., chapter 2, in Ozonation in Organic Chemistry. Volume II: Nonolefinic Compounds. New York: Academic Press. 1982. ISBN 0120731029.
- ^ Cremer, D. (2001). "The Ozonolysis of Acetylene-A Quantum Chemical Investigation". J. Am. Chem. Soc. 123 (25): 6127–6141. doi:10.1021/ja010166f.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help)

