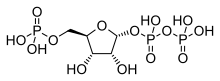
Phosphoribosyl pyrophosphate
| |
| Names | |
|---|---|
| IUPAC name
α-D-Ribofuranose 1′-(trihydrogen diphosphate) 5′-(dihydrogen phosphate)
| |
| Systematic IUPAC name
(2R,3R,4S,5R)-3,4-Dihydroxy-5-[(phosphonooxy)methyl]oxolan-2-yl trihydrogen diphosphate | |
| Other names
5-phospho-α-D-ribose 1-diphosphate
PRPP | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| DrugBank | |
| MeSH | Phosphoribosyl+pyrophosphate |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C5H13O14P3 | |
| Molar mass | 390.07 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Phosphoribosyl pyrophosphate (PRPP) is a pentose phosphate. It is a biochemical intermediate in the formation of purine nucleotides via inosine-5-monophosphate, as well as in pyrimidine nucleotide formation. Hence it is a building block for DNA and RNA.[1][2][3] The vitamins thiamine[4] and cobalamin,[5] and the amino acid tryptophan also contain fragments derived from PRPP.[6] It is formed from ribose 5-phosphate (R5P) by the enzyme ribose-phosphate diphosphokinase:[7]
It plays a role in transferring phospho-ribose groups in several reactions, some of which are salvage pathways:[8]
In de novo generation of purines, the enzyme amidophosphoribosyltransferase acts upon PRPP to create phosphoribosylamine.[2] The histidine biosynthesis pathway involves the reaction between PRPP and ATP, which activates the latter to ring cleavage. Carbon atoms from ribose in PRPP form the linear chain and part of the imidazole ring in histidine.[15][16][17] The same is true for the biosynthesis of tryptophan, with the first step being N-alkylation of anthranilic acid catalysed by the enzyme anthranilate phosphoribosyltransferase.[15][18][19]
Increased PRPP
[edit]Increased levels of PRPP are characterized by the overproduction and accumulation of uric acid leading to hyperuricemia and hyperuricosuria. It is one of the causes of gout.[20]
Increased levels of PRPP are present in Lesch–Nyhan Syndrome. Decreased levels of hypoxanthine guanine phosphoribosyl transferase (HGPRT) causes this accumulation, as PRPP is a substrate used by HGPRT during purine salvage.[21]
See also
[edit]References
[edit]- ^ R. Caspi (2009-01-13). "Pathway: 5-aminoimidazole ribonucleotide biosynthesis I". MetaCyc Metabolic Pathway Database. Retrieved 2022-02-02.
- ^ a b Zhang, Y.; Morar, M.; Ealick, S.E. (2008). "Structural biology of the purine biosynthetic pathway". Cellular and Molecular Life Sciences. 65 (23): 3699–3724. doi:10.1007/s00018-008-8295-8. PMC 2596281. PMID 18712276.
- ^ Gupta, Rani; Gupta, Namita (2021). "Nucleotide Biosynthesis and Regulation". Fundamentals of Bacterial Physiology and Metabolism. pp. 525–554. doi:10.1007/978-981-16-0723-3_19. ISBN 978-981-16-0722-6. S2CID 234897784.
- ^ Chatterjee, Abhishek; Hazra, Amrita B.; Abdelwahed, Sameh; Hilmey, David G.; Begley, Tadhg P. (2010). "A "Radical Dance" in Thiamin Biosynthesis: Mechanistic Analysis of the Bacterial Hydroxymethylpyrimidine Phosphate Synthase". Angewandte Chemie International Edition. 49 (46): 8653–8656. doi:10.1002/anie.201003419. PMC 3147014. PMID 20886485.
- ^ R. Caspi (2019-09-23). "Pathway: 5-hydroxybenzimidazole biosynthesis (anaerobic)". MetaCyc Metabolic Pathway Database. Retrieved 2022-02-10.
- ^ Mehta, Angad P.; Abdelwahed, Sameh H.; Fenwick, Michael K.; Hazra, Amrita B.; Taga, Michiko E.; Zhang, Yang; Ealick, Steven E.; Begley, Tadhg P. (2015). "Anaerobic 5-Hydroxybenzimidazole Formation from Aminoimidazole Ribotide: An Unanticipated Intersection of Thiamin and Vitamin B12 Biosynthesis". Journal of the American Chemical Society. 137 (33): 10444–10447. doi:10.1021/jacs.5b03576. PMC 4753784. PMID 26237670.
- ^ Li, Sheng; Lu, Yongcheng; Peng, Baozhen; Ding, Jianping (January 2007). "Crystal structure of human phosphoribosylpyrophosphate synthetase 1 reveals a novel allosteric site". Biochemical Journal. 401 (1): 39–47. doi:10.1042/BJ20061066. PMC 1698673. PMID 16939420.
- ^ R. Caspi (2022-02-15). "5-phospho-α-D-ribose 1-diphosphate". MetaCyc Metabolic Pathway Database. Retrieved 2022-02-15.
- ^ Silva, Carlos H. T. P.; Silva, Marcio; Iulek, Jorge; Thiemann, Otavio H. (2008). "Structural Complexes of Human Adenine Phosphoribosyltransferase Reveal Novel Features of the APRT Catalytic Mechanism". Journal of Biomolecular Structure and Dynamics. 25 (6): 589–597. doi:10.1080/07391102.2008.10507205. PMID 18399692. S2CID 40788077.
- ^ a b Finette, Barry A.; Kendall, Heather; Vacek, Pamela M. (2002). "Mutational spectral analysis at the HPRT locus in healthy children". Mutation Research/Fundamental and Molecular Mechanisms of Mutagenesis. 505 (1–2): 27–41. doi:10.1016/S0027-5107(02)00119-7. PMID 12175903.
- ^ Vinitsky, A.; Grubmeyer, C. (1993). "A new paradigm for biochemical energy coupling. Salmonella typhimurium nicotinate phosphoribosyltransferase". Journal of Biological Chemistry. 268 (34): 26004–26010. doi:10.1016/S0021-9258(19)74485-8. PMID 7503993.
- ^ González-Segura, Lilian; Witte, John F.; McClard, Ronald W.; Hurley, Thomas D. (2007). "Ternary Complex Formation and Induced Asymmetry in Orotate Phosphoribosyltransferase". Biochemistry. 46 (49): 14075–14086. doi:10.1021/bi701023z. PMID 18020427.
- ^ Selwood, Trevor; Jaffe, Eileen K. (2012). "Dynamic dissociating homo-oligomers and the control of protein function". Archives of Biochemistry and Biophysics. 519 (2): 131–143. doi:10.1016/j.abb.2011.11.020. PMC 3298769. PMID 22182754.
- ^ Krenitsky, Thomas A.; Neil, Shannon M.; Miller, Richard L. (1970). "Guanine and Xanthine Phosphoribosyltransfer Activities of Lactobacillus casei and Escherichia coli". Journal of Biological Chemistry. 245 (10): 2605–2611. doi:10.1016/S0021-9258(18)63113-8.
- ^ a b Voet, Donald (2016). Fundamentals of biochemistry : life at the molecular level. Judith G. Voet, Charlotte W. Pratt (Fifth ed.). Hoboken, NJ. ISBN 978-1-118-91840-1. OCLC 910538334.
{{cite book}}: CS1 maint: location missing publisher (link) - ^ R. Caspi (2008-10-10). "Pathway: L-histidine biosynthesis". MetaCyc Metabolic Pathway Database. Retrieved 2022-02-17.
- ^ Stepansky, A.; Leustek, T. (2006). "Histidine biosynthesis in plants". Amino Acids. 30 (2): 127–142. doi:10.1007/s00726-005-0247-0. PMID 16547652. S2CID 23733445.
- ^ C.A. Fulcher (2010-02-12). "Pathway: L-tryptophan biosynthesis". MetaCyc Metabolic Pathway Database. Retrieved 2022-02-17.
- ^ Crawford, Irving P. (1989). "Evolution of a Biosynthetic Pathway: The Tryptophan Paradigm". Annual Review of Microbiology. 43: 567–600. doi:10.1146/annurev.mi.43.100189.003031. PMID 2679363.
- ^ Elliott, Katherine; Fitzsimons, David W. (16 September 2009). Purine and Pyrimidine Metabolism. John Wiley & Sons. pp. 143–158. ISBN 9780470717981.
- ^ Cakmakli, Hasan F.; Torres, Rosa J.; Menendez, Araceli; Yalcin-Cakmakli, Gul; Porter, Christopher C.; Puig, Juan Garcia; Jinnah, H.A. (2019). "Macrocytic anemia in Lesch–Nyhan disease and its variants". Genetics in Medicine. 21 (2): 353–360. doi:10.1038/s41436-018-0053-1. PMC 6281870. PMID 29875418.

