Polymer capacitor
This article may require copy editing for grammar, style, cohesion, tone, or spelling. (September 2015) |


A polymer capacitor, or more accurately a polymer electrolytic capacitor, is an electrolytic capacitor (e-cap) with a solid electrolyte of a conductive polymer. It is based on the use of an anode metal and the combination of a polymer electrolyte together with a liquid electrolyte. There are three different types:
- Polymer tantalum electrolytic capacitor
- Polymer aluminum electrolytic capacitor
- Hybrid polymer capacitor
Polymer niobium electrolytic capacitors are not yet in production.
Polymer electrolytic capacitors in rectangular SMD chip style are available with a sintered tantalum anode or with stacked aluminum anode foils. In cylindrical SMDs (V-chips) style or as radial leaded versions (single-ended) they are available only with wound aluminum anode foils.
Polymer capacitors are characterized by particularly low internal equivalent series resistances (ESR) and high ripple current ratings. Their electrical parameters have similar temperature dependence, reliability and service life compared to solid tantalum capacitors, but have a very much better temperature dependence and a considerably longer service life than aluminum electrolytic capacitors with non-solid electrolytes. In general polymer capacitors have a higher leakage current rating than the other solid or non-solid electrolytic capacitors.
Polymer electrolytic capacitors are also available in a hybrid construction. The hybrid polymer aluminum electrolytic capacitors may have either a solid polymer electrolyte or a liquid electrolyte. These types are characterized by low ESR values but have low leakage currents and are insensitive to transient,[1] however they have a temperature-dependent service life similar to non-solid e-caps.
Polymer electrolytic capacitors are mainly used as power supplies of integrated electronic circuits as buffer, bypass and decoupling capacitors, especially in devices with flat or compact design. Thus they compete with (MLCC), but offer higher capacitance values than MLCC capacitors. The also they display no microphonic effect.
History
Aluminum electrolytic capacitors with liquid electrolytes were invented in 1896 by Charles Pollak, see electrolytic capacitor.
Tantalum electrolytic capacitors with solid manganese dioxide electrolytes were invented by Bell Laboratories in the early 1950s, as a miniaturized and more reliable low-voltage support capacitor to complement the newly invented transistor,[2][3] see Tantalum capacitor. The first tantalum capacitors (Ta-caps) with solid manganese dioxide electrolytes had 10 times better conductivity and a higher ripple current load than earlier types of non-solid electrolyte capacitors (e-caps). Additionally, unlike standard e-caps, the equivalent series resistance (ESR) of Ta-caps is stable in varying temperatures.

During the 1970s, the increasing digitization of electronic circuits came with decreasing operating voltages, and increasing switching frequencies and ripple current loads. This had consequences for power supplies and their electrolytic capacitors. There was a need for capacitors with lower ESR and lower equivalent series inductance (ESL) for bypass and decoupling capacitors used in power supply lines,[4] seeRole of ESR, ESL and capacitance. A breakthrough came in 1973, with the discovery by A. Heeger and F. Wudl[5] of an organic conductor, the charge-transfer salt TCNQ. TCNQ (7,7,8,8-tetracyanoquinodimethane or N-n-butyl isoquinolinium in combination with TTF (Tetrathiafulvalene)) is a chain molecule of almost perfect one-dimensional structure that has a 10-fold better conductivity along the chains than does manganese dioxide, and has a 100-fold better conductivity than non-solid electrolytes.

The first aluminum electrolytic capacitors to use the charge transfer salt TTF-TCNQ as a solid organic electrolyte was the OS-CON series offered 1983 from Sanyo. These wound, cylindrical capacitors provided an improvement in conductivity (increased or decreased?) of the electrolyte by a factor of 10 as compared with the manganese dioxide electrolyte[6][7][8] Although Sanyo was the only manufacturer, these capacitors were used worldwide in many devices for applications which required the lowest possible ESR or highest possible ripple current. The technological advance was great, only one of these new OS-CON e-caps could replace three much more bulky "wet" e-caps or two Ta-caps. The success of these capacitors was great and by 1995, the Sanyo OS-CON became the preferred decoupling capacitor for the Pentium Processor used in IBM PC’s.
The Sanyo OS-CON e-caps with TCNQ electrolyte was sold in 2010 to Panasonic after which Panasonic replaced the TCNQ salt in OS-CON capacitors with a conducting polymer but sold the capacitors under the same brand name.
The next step in ESR reduction was the development of conducting polymers by Alan J. Heeger, Alan MacDiarmid and Hideki Shirakawa in 1975.[9] The conductivity of conductive polymers such as polypyrrole (PPy) [10] or PEDOT [11] is better than that of TCNQ by a factor of 100 to 500, and close to the conductivity of metals.
In 1988 the first polymer electrolyte e-cap, "APYCAP" with PPy polymer electrolyte, was launched by the Japanese manufacturer Nitsuko with the intention of revolutionizing the industry [12] Nitsuko did not have great success with these capacitors, in part because they were not available in SMD versions.
In 1991 Panasonic came on the market with its "SP-Cap",[13] called polymer aluminum electrolytic capacitors. These aluminum electrolytic capacitors with polymer electrolytes reached very low ESR values that were directly comparable to ceramic multilayer capacitors (MLCCs). They were still less expensive than tantalum capacitors and with their flat design useful in compact devices such as laptops and cell phones they competed with tantalum chip capacitors as well.
Tantalum electrolytic capacitors with PPy polymer electrolyte cathode followed three years later. In 1993 NEC introduced its SMD polymer tantalum electrolytic capacitors, called "NeoCap". In 1997 Sanyo followed with the "POSCAP" polymer tantalum chips.
A new conductive polymer for tantalum polymer capacitors was presented by Kemet at the "1999 Carts" conference.[14] This capacitor used the newly developed organic conductive polymer PEDT (Poly(3,4-ethylenedioxythiophene)), also known as PEDOT (trade name Baytron®) [15]
Two years later at the 2001 APEC Conference, Kemet introduced PEDOT polymer aluminum e-caps to the market.[16] The AO-Cap series included SMD capacitors with stacked anode in "D" size with heights from 1.0 to 4.0 mm, in competition to the Panasonic SP-Caps using polypyrrole at that time.
A disadvantage of polymer electrolytic capacitors is their relatively high leakage current. Because the conductive polymer electrolyte provides no oxygen for self-healing processes the dielectric can be weakened, f. e. after soldering resulting in a high leakage current. For this reason around the turn of the millennium hybrid polymer capacitors were developed, which have in addition to the polymer electrolyte a liquid electrolyte connecting the polymer layers covering the dielectric layer and the cathode foil.[1][17] The non-solid electrolyte provide oxygen for self-healing purposes to reduce the leakage current. In 2001, NIC launched a hybrid polymer e-cap to replace a polymer type at lower price and with lower leakage current. As of 2015 the hybrid polymer capacitors are available from much more than only one manufacturer.
Application basics
Role of ESR, ESL and capacitance
The predominant application range of electrolytic capacitors, also of polymer capacitors is in the range of power supplies. Here they cause behind the rectifying smoothing of the rectified AC voltage or interference suppression and buffer or stabilize the DC voltage at a sudden power demand of the subsequent circuit. This type of capacitors is named backup-, bypass- or decoupling capacitors.[18] In these applications, in addition to the size, are the capacitance, the impedance Z, the ESR, and the inductance ESL important electrical characteristics for the functionality of these capacitors in the circuit,

The search for better capacitors, which resulted in the development of the polymer electrolyte capacitors, began with the digitalization of electronic equipment. The increasing functions in integrated circuits, the development of switching power supplies with higher frequencies, and the development of "on-board" DC/DC converter had changed the requirements on the capacitors. Frequencies had gone higher and higher, supply voltages had decreased and supply currents had increased. Due to these requirements the decoupling capacitors for new developments must have lower ESR values, which at that times only could be realized with larger case sizes, because larger types has lower ESR. This trend was against the industrial trend of miniaturizing. Only solid tantalum capacitors could reach the increasing requirements, however they are much more expensive than Al e-caps.
The reason how the ESR influences the functionality of an integrated circuit is simple. If the circuit, f. e. a microprocessor, has a sudden power demand, the supply voltage drops by ESL, ESR and capacitance charge loss. Because in case of a sudden current demand the voltage of the power line drops with:
- ΔU = ESR • I
As an example of orders of magnitude, which must be considered in electronic circuits, the following values may serve:[4]
The supply voltage of a processor is 3 V, with a tolerance of 10% (200 mV) and the supply current is a maximum of 10 A. In case of a sudden power demand the ESR of the capacitor determines primarily the voltage drop by:
- ESR = U / I = 0.3 V / 10 A = 30 milliohms.
This means that the ESR of a capacitor in the power supply of a CPU must be less than 30 mΩ, otherwise it comes to malfunction in the circuit.
Similar rules are valid for capacitance and ESL. The specific capacitance could be increased over the years by higher etched anode foils respectively by smaller and finer tantalum powder grains by a factor of 10 to 15 and could follow the trend of miniaturizing. The ESL challenge has led to the stacked foil versions of polymer Al e-caps. However, for lowering the ESR only the development of new, solid conductive materials, first TCNQ, after than the conductive polymers, which led to the development of the polymer electrolyte capacitors with their very low ESR values, the ESR challenge of digitization of electronic circuits could be accepted.
Electrolytic capacitors - basics
Anodic oxidation

Electrolytic capacitors use a chemical feature of some special metals, earlier called "valve metals", on which by anodic oxidation (forming) an insulating oxide layer originates and serves as dielectric. By applying a positive voltage to the anode material in an electrolytic bath an oxide barrier layer with a thickness corresponding to the applied voltage can be formed. This oxide layer acts as the dielectric in an electrolytic capacitor. After forming a dielectric oxide on the rough anode (+) structure, a counter electrode has to match the rough insulating oxide surface. This is accomplished by the electrolyte, which acts as the cathode (-) electrode of an electrolytic capacitor.
Main difference between the polymer capacitors is the anode material and its oxide used as the dielectric:
- polymer electrolytic capacitors use high purity sintered tantalum powder as an anode with tantalum pentoxide (Ta2O5) as a dielectric and
- Polymer aluminum electrolytic capacitors use a high purity and electrochemically etched (roughened) aluminum foil as an anode with aluminum oxide (Al2O3) as the dielectric
The properties of the aluminum oxide layer compared with tantalum pentoxide dielectric layer are given in the following table:
| Anode- material |
Dielectric | Permittivity ε |
Oxide structure |
Breakdown voltage (V/µm) |
Dielectric layer thickness (nm/V) |
|---|---|---|---|---|---|
| Tantalum | Tantalum pentoxide Ta2O5 | 27 | amorphous | 625 | 1.6 |
| Aluminum | Aluminum oxide Al2O3 | 9.6 | amorphous | 710 | 1.4 |
| crystalline | 1000 | 1.0 |

Every electrolytic capacitor in principle forms a "plate capacitor" whose capacitance is greater, the larger the electrode area A and the permittivity ε, and the thinner the thickness (d) of the dielectric.
The capacitance is proportional to the product of the area of one plate multiplied with the permittivity, divided by the thickness of the dielectric.
The dielectric thickness of electrolytic capacitors is very slight, in the range of nanometers per volt. On the other hand, the voltage strengths of these oxide layers are quite high. With this very thin dielectric oxide layer combined with a sufficient high dielectric strength the electrolytic capacitors can already achieve a high volumetric capacitance. This is one reason for the high capacitance values of electrolytic capacitors compared to conventional capacitors. All etched or sintered anodes have a much higher surface compared to a smooth surface of the same area or the same volume. That increases the later capacitance value, depending on the rated voltage, by a factor of up to 200 for non-solid aluminum electrolytic capacitors as well as for solid tantalum electrolytic capacitors.[20][21][22] The large surface compared to a smooth one is the second reason for the relatively high capacitance values of electrolytic capacitors compared with other capacitor families.
All electrolytic capacitors have one special advantage. Because the forming voltage defines the oxide layer thickness, the voltage proof of the later electrolytic capacitor can be produced very simply for the desired rated value. Therefore, the volume of a capacitor is defined by the product of capacitance and voltage, the so-called "CV product".
Comparing the dielectric constants of tantalum and aluminum oxide materials, tantalum pentoxide has a permittivity approximately 3-fold higher than aluminum dioxide. Tantalum electrolytic capacitors therefore theoretically could be smaller than aluminum electrolytic capacitors with the same capacitance and rated voltage values. For real tantalum electrolytic capacitors, the oxide layer thicknesses are much thicker than the rated voltage of the capacitor actually requires. This is done for safety reasons to avoid shorts coming from field crystallization.[23] For this reason the real differences of sizes that derive from the different permittivities, are partially ineffective.
Electrolytes
The most important electrical property of an electrolyte in an electrolytic capacitor is its electrical conductivity. The electrolyte forms the counter electrode, of the electrolytic capacitor, the cathode. Since the roughened structures of the anode surface continue in the structure of the oxide layer, the dielectric, the cathode must adapt precisely to the roughened structure. With a liquid, as in the conventional "wet" e-caps that is easy to reach. In the case of polymer electrolytic capacitors in which a solid conductive polymer forms the electrolyte, which obtained its conductivity only by a chemical process of polymerization, this is much more difficult to achieve. However, the benefits of a solid polymer electrolyte, the significantly lower ESR of the capacitor and the low temperature dependence of the electrical parameters, in many cases justify the additional production steps as well as higher costs.
Conducting salt TCNQ electrolyte

Electrolytic capacitors with the charge transfer salt tetracyanoquinodimethane TCNQ as electrolyte, formerly produced by Sanyo with the trade name "OS-CON", in the true sense of the term "polymer" were no "polymer capacitors". TCNQ electrolytic capacitors are mentioned here to point out the danger of confusion with 'real' polymer capacitors, which are sold nowadays under the same trade name OS-CON. The original OS-CON capacitors with TCNQ electrolyte sold by the former manufacturer Sanyo has been discontinued with the integration of Sanyo capacitor businesses by Panasonic 2010.[24] Panasonic keep the trade name OS-CON but change the TCNQ electrolyte into a conductive polymer electrolyte (PPy).[25] Electrolytic capacitors with TCNQ electrolyte are not available anymore.
Polymer electrolyte
A polymer is formed by a chemical reaction, the polymerization. This is a reaction where monomers are continuously attached to a growing polymer.[26] Usually polymers are electrically insulators, at best, semiconductors. For use as an electrolyte in electrolytic capacitors, electrical conductive polymers are needed. The conductivity of a polymer is obtained by conjugated double bonds which permit free movement of charge carriers in the doped state. As charge carriers serve electron holes. That means, the conductivity of conducting polymers, which is nearly comparable with metallic conductors, only starts when the polymers are doped oxidatively or reductively.
The requirements for a polymer electrolyte prior to the polymerization are manifold. It must be able to penetrate the roughened anode deep into the finest structures in order to form a most complete, homogeneous layer, because only anode oxide sections covered by the electrolyte contribute to the total capacitance. For this the precursors of the polymer has to consist of very small base materials that can penetrate even the smallest pores. The size of this precursors are the limiting factor in the size of the pores in the etched of aluminum anode foils or of the size of tantalum powder. The rate of polymerization must be controlled for capacitor manufacturing, too rapid polymerization would not lead to a complete coverage of the anode, a too slow polymerization could increase the cost of production. Neither the precursors nor the polymer or its residues may the oxide on the anode attack chemically or mechanically. When finished as electrolyte it should have a high stability over a wide temperature range and maintain them over a long period. The polymer film is not only the counter electrode of the capacitor, it also protects the dielectric even against external influences such as the direct contact of graphite in this capacitors, which are provided with a cathode contact via graphite and silver.
In the today (2015) produced polymer electrolytic capacitors, two different conductive polymers are in use. These are polypyrrole, abbreviated PPy,[27] the first used conductive polymer in electrolyte capacitors, and polythiophene, abbreviated PEDOTor PEDT[28][29]
Polypyrrole PPy


Polypyrrole (PPy) is a type of conducting polymer formed by oxidative polymerization of pyrrole. A suitable oxidizing agent is iron (III) chloride (FeCl3). Water, methanol, ethanol, acetonitrile and other polar solvents may be used for the synthesis of PPy.[31] As a solid conducting polymer electrolyte It reaches conductivities up to 100 S/m.
Polypyrrole was the first conductive polymer used in polymer electrolytic capacitors, first into polymer Al-e-caps, a few years later also in polymer Ta-e-caps. The problem with the in situ polymerization of PPy was the rate of polymerization. When pyrrole is mixed with the desired oxidizing agents at room temperature, the polymerization reaction begins immediately. Thus polypyrrole begins to form, before the chemical solution can get into the pores of the anode. The polymerization rate can be controlled by two methods, by cryogenic cooling or by electrochemically polymerization. The method of cooling of the PPy basic substances to very low temperatures requires a very great technical effort and is unfavorable for mass production. In the electrochemical polymerization at first an auxiliary electrode layer on the dielectric has to be applied and to be connected to the anode.[29] For this purpose, ionic dopants are added to the basic substances of the polymer, forming a conductive layer on the surface of the dielectric during the first impregnation. During the following impregnation cycles, the in-situ polymerization can be time-controlled by the current flow after applying a voltage between the anode and cathode. With this method a fine and stable polypyrrole film on the dielectric oxide layer of the anode can be realized.[32] However, both methods of in situ polymerization are complex and requires a multiple repetition of polymerization steps which makes the manufacturing cost intensive.
The polypyrrole electrolyte has two fundamental disadvantages. It is toxic in the production of capacitors and becomes unstable at the higher soldering temperatures required for soldering with lead-free solders.[29]
Polythiopene PEDOT and PEDOT:PSS


Poly(3,4-ethylenedioxythiophene), abbreviated PEDOT or PEDT[28] is a conducting polymer based on 3,4-ethylenedioxythiophene or EDOT monomer. PEDOT polarized by the oxidation of EDOT with catalytic amounts of iron (III) sulfate. The re-oxidation of iron is given by Sodium persulfate.[33] Advantages of this polymer are optical transparency in its conducting state, non toxic, stable up to temperatures of 280 °C and reaches a conductivity up to 500 S/m.[29] Especially due to its heat resistance polymer capacitors can be manufactured, which can withstand the increased soldering temperatures of lead-free soldering. Additional this capacitors have better ESR values as polymer e-caps with PPy electrolyte.[29]
The difficult methods of in situ polymerization of PEDOT in the anodes of the capacitors initially were the same as with polypyrrole. This changed with the development of pre-polymerized dispersions of PEDOT in which the anodes of capacitors at room temperature simple could be dipped and then dried. For this purpose, the PEDOT chemicals is added with sodium polystyrene sulfonate (PSS) and dissolved in water.[34] The complete polymer layer on the dielectric is then composed of pre-polymerized particles from the dispersion. These dispersions are known as PEDOT: PSS, trade names Baytron P®[35] and Clevius™.[36] The good properties of the PEDOT polymer as electrolyte for e-caps is not lost by this.[37][38]
This PEDOT:PSS dispersions are available in different variants. For capacitors with high capacitance values with high-roughened aluminum anode foils or fine-grained tantalum powders, dispersions having very small particle sizes are offered. The average size of these pre-polymerized particles is about 30 nm in order impregnate even the finest capillaries in the anode structures of the capacitors. Another variant of a PEDOT:PSS dispersion has been developed with larger pre-polymerized particles leading to a relatively thick polymer layer in order to make an enveloping protection of the capacitive cell of rectangular Ta and Al polymer capacitors against mechanical and electrical stress.[29][36]
With PEDOT:PSS dispersions produced polymer aluminum electrolytic capacitors are well suited to reach higher rated voltage values of 200 V[39] and 250 V.[40] In addition, the leakage current values of the polymer electrolytic capacitors, which are produced with these dispersions, are significantly lower than for polymer capacitors having polymerized in-situ polymer layers. Beneath to the better ESR values, higher temperature stability and lower leakage current values, however, the ease of manufacture of polymer capacitors with the pre-polymerized PEDOT:PSS dispersions, which in already only three dips of immersion have an almost complete coverage of the dielectric with a conducting polymer layer,[34] which make the production of polymer capacitors significantly less expensive, is the main advantage of this PEDOT:PSS dispersions.
Hybrid electrolyte
Relatively new are hybrid polymer aluminum electrolytic capacitors. They combine a coating of the roughened and oxidized aluminum anode structure with a conductive polymer together with a liquid electrolyte. The liquid electrolyte is soaked in the separator (spacer) and achieves with its ion conductivity the electrical contact between the both polymer layers covering the dielectric and on the cathode foil. The liquid electrolyte can supply the oxygen for self-healing processes of the capacitor, which reduces the leakage current, so that values such as in conventional "wet" the electrolytic capacitor can be achieved. In addition the safety margin for the required oxide thickness for a desired rated voltage can be reduced.
The effects of the liquid electrolyte on ESR and temperature characteristics are relatively low. By using appropriate organic electrolytes and a good sealing of the capacitors a long service life can be achieved.[17][41]
Types and styles
Based on the used anode metal and the combination of a polymer electrolyte together with a liquid electrolyte, there are three different types:
- Polymer tantalum electrolytic capacitor
- Polymer aluminum electrolytic capacitor
- Hybrid polymer aluminum electrolytic capacitor
These three different types, also known as families, are produced in two different styles:
- as a rectangular SMD chip, usually molded with a plastic case, available with sintered tantalum anode or with stacked aluminum anode foils and
- in a cylindrical style with a wound cell in a metal case, available as cylindrical SMDs (V-chips) style or as radial leaded versions (single-ended)
- Styles of polymer electrolytic capacitors
-
Rectangular SMD chips are available with sintered tantalum anode or with stacked aluminum anode foils
-
Cylindrical styles with a wound cell in a metal case are available as SMDs (V-chips) or as radial leaded versions (single-ended) for polymer or hybrid polymer aluminum capacitors
A comparison of the at the time (2015) deliverable capacitance and voltage values shows the table below the basic #Parameter comparization
Rectangular chip style
The development of polymer tantalum electrolytic capacitors in the early 1990s coincided with the development of devices with a flat design as mobile phones and laptops in SMD assembly technology. The rectangular base surface is utilized to the maximum mounting space, which is not the case with round base surfaces. In addition, the sintered cell can be manufactured so that the finished SMD component has a desired height. In many cases this is the height of the processors and other semiconductor devices. Typical is for example the height of about 0.8 to 4 mm.
Polymer tantalum chip capacitors
Polymer tantalum electrolytic capacitors are essentially tantalum capacitors in which the electrolyte is a conductive polymer instead of manganese dioxide, see also tantalum capacitor#Materials, production and styles Tantalum capacitors are manufactured from a powder of relatively pure elemental tantalum metal.[42][43][44]
The powder is compressed around a tantalum wire, the anode connection, to form a "pellet". This pellet/wire combination is subsequently vacuum sintered at high temperature (typically 1200 to 1800 °C) which produces a mechanically strong anode pellet. During sintering, the powder takes on a sponge-like structure, with all the particles interconnected into a monolithic spatial lattice. This structure is of predictable mechanical strength and density, but is also highly porous, producing a large anode surface area.
The dielectric layer is then formed over all the tantalum particle surfaces of the anode by the electrochemical process of anodization or forming. To achieve this, the "pellet" is submerged into a very weak solution of acid and DC voltage is applied. The total dielectric thickness is determined by the final voltage applied during the forming process. Thereafter, the oxidized sintered block is impregnated with the precursors of the polymer, which then polymerized in a chemical process into the conductive polymer as a thin layer on the dielectric, the counter electrode. This polymerized pellet now is successively dipped into conducting graphite and then silver to provide a good connection to the conducting polymer. This layers achieves the cathode connection of the capacitor. The capacitive cell then is generally molded by a synthetic resin.
- Basic construction of a polymer tantalum capacitor
-
Layer structure of a polymer tantalum capacitor with graphit/silver cathode connection
-
Basic cross-section of a rectangular polymer tantalum chip capacitor
-
Rectangular polymer tantalum chip capacitor
Polymer tantalum electrolytic capacitors have ESR values that are approximately only 1/10 of the value of tantalum electrolytic capacitors with manganese dioxide electrolyte of the same size. By a multi-anode technique in which several anode blocks are connected in parallel in one case, the ESR value can be reduced again. The advantage of the multi-anode technology in addition to the very low ESR values is the lower inductance ESL, whereby the capacitors are suitable for higher frequencies.
The disadvantage of all polymer tantalum capacitors is the higher leakage current, which is approximately by a factor of 10 higher compared to the capacitors with manganese dioxide electrolyte. Polymer SMD tantalum electrolytic capacitors are available up to a size of 7.3x4.3x4.3 mm (length x width x height) with a capacity of 1000 µF at 2.5 V. They cover temperature ranges from −55 °C to +125 °C and are available in rated voltage values from 2.5 to 63 V.
New designs – lowering ESR and ESL
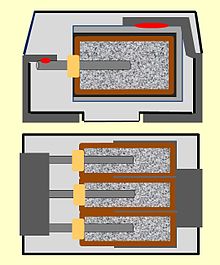
Objective for all polymer capacitors is to reduce the ESR and, if possible, the ESL by using low ohmic polymer electrolyte. However, constructive measures can have also a major impact on the electrical parameters of capacitors. Smaller ESR values can be achieved for example by parallel connection of several conventional capacitor cells in one case. Three parallel capacitors with an ESR of 60 mΩ each have a resulting ESR of 20 mΩ. This technology is called "multi-anode" construction and will be used at polymer tantalum capacitors.[45][46] In this construction up to six individual anodes in one case are connected. This design is offered as polymer tantalum chip capacitors as well as lower expensive tantalum chip capacitors with MnO2 electrolyte. Multi-anode polymer tantalum capacitors have ESR values on the single-digit milliohm range.
But not only the ESR plays a role in the use of polymer tantalum capacitors. Through simple constructive changes the parasitic inductance of the capacitor, the ESL, also can be lowered. Since the length of the leads inside the capacitor case has a large amount of the total ESL the inductance of the capacitor can be reduced by reducing the length of the internal leads by asymmetric sintering of the anode lead. This technique is called "face-down" construction. Due to the lower ESL of this face-down construction, the resonance of the capacitor is shifted to higher frequencies, which take into account the faster load changes of digital circuits with ever-higher switching frequencies.[47]

.
Polymer tantalum chip capacitors with these new design enhancements, that both the ESR and the ESL decreased reaching properties, approaching ever closer to those of MLCC capacitors.
Polymer aluminum chip capacitors
The rectangular polymer aluminum electrolytic capacitors are aluminum electrolytic capacitors with one or more layered aluminum anode foils and a conductive polymer as electrolyte. The layered anode foils are at one side contacted with each other, this block is anodically oxidized to achieve the dielectric, and the block is impregnated with the precursors of the polymer, which then polymerized in a chemical process into the conductive polymer as a thin layer on the dielectric, the counter electrode. Like for polymer tantalum capacitors this polymerized block now is successively dipped into conducting graphite and then silver to provide a good connection to the conducting polymer. This layers achieves the cathode connection of the capacitor. The capacitive cell then generally is molded by a synthetic resin.
- Basic construction of a polymer aluminum capacitor with layered anode stripes
-
Layer structure of a polymer aluminum capacitor with graphit/silver cathode connection
-
Basic cross-section of a rectangular polymer aluminum chip capacitor
-
Rectangular polymer aluminum chip capacitor. The external appearance has no indication of the used internally anode material.
The layered anode foils in the rectangular shaped polymer aluminum chip capacitors are electrically parallel connected single capacitors. Thus, the ESR and ESL values are connected in parallel and are correspondingly smaller. Due to this design the advantage of the polymer electrolyte is enhanced, achieving not only very low ESR values, but also have a lower inductance ESL. Thus, these capacitors are suitable for higher frequencies. This polymer capacitors are simply said "faster", based on the switching speed.
The rectangular polymer aluminum chip capacitors are available in the "D"-case with 7,3x4,3&bsp;mm and heights of round 2…4 mm. Thus they provide mechanical a competitive type identical to corresponding polymer tantalum capacitors with comparable electrical values[48]
Comparing identical polymer-aluminum, and polymer-tantalum chip capacitors (#Comparization of electrical parameters) shows that in reality the different permittivities of aluminum oxide and tantalum pentoxide has little impact on the specific capacity. This is due to the different safety margins in the production of the oxide films. For polymer tantalum capacitors an oxide layer thickness is generated which corresponds to approximately four times the rated voltage, wherein the polymer aluminum capacitors only have about twice the rated voltage.
Cylindrical (radial) style
Cylindrical polymer aluminum capacitors based on the technique of wound aluminum electrolytic capacitors with liquid electrolytes, see Aluminum electrolytic capacitor#Production. They are only available with aluminum as the anode material.
Cylindrical polymer aluminum capacitors are intended for larger capacitance values with respect to the rectangular polymer capacitors. Due to their design, they may vary in height on a given surface mounting area so that larger capacitance values can be achieved by higher case without increasing the mounting surface. This is a great advantage for printed circuit boards at not limited height.
Cylindrical polymer aluminum capacitors
The cylindrical polymer capacitors are made of two aluminum foils as in the conventional aluminum electrolytic capacitors, an etched and formed anode and a cathode foil, which are mechanically separated from each other by a separator and wound into a winding. The winding is impregnated with the precursors of the polymer, which then polymerized to form the conductive polymer as a thin layer on the dielectric and on the cathode foil, and as conducting connections through the separator connecting electrically both layers. The winding then is built into an aluminum case and sealed with a rubber sealing. For the SMD version (Vertical chip= V-chip) of the case is provided with a bottom plate.
- Design principles of cylindrical polymer aluminum capacitors
-
Winding of an aluminum electrolytic capacitor
-
Cross-sectional view of the capacitive cell of a wound polymer aluminum capacitor with polymer electrolyte
-
Cylindrical polymer aluminum capacitors with wound cell in cylindrical metal case, in radial leaded (single-ended) and SMD style (V-chip)
The cylindrical polymer aluminum electrolytic capacitors are less expensive than corresponding polymer tantalum capacitors in a given CV value (capacitance x rated voltage). They are available up to a size of 10x13 mm (diameter x height) with a CV value of 3900 µF/2.5 V[49] They can cover temperature ranges from -55 °C to +125 °C and are available in nominal voltage values from 2.5 to 200 V.[39]
Unlike the so-called. "wet" Al electrolytic capacitors the case of polymer Al capacitors don’t have a predetermined vent (notch) in the bottom of the case. Since the polymer electrolyte capacitor in case of short circuit don’t have a gas formation, there don’t accrue a gas pressure in the case, therefore is not a breaking point required.
Hybrid polymer aluminum capacitors

Hybrid polymer capacitors are only available in the cylindrical style with wound aluminum anode and cathode foils, separated by a spacer, leaded in the radial (single-ended) design or with the additional base plate in SMD version (V-chip). Their construction thus corresponds to the above-described cylindrical polymer aluminum capacitors. The polymerized thin layer of the polymer electrolyte covers both the roughened structure of the dielectric and the surface of the cathode foil. The main difference compared with cylindrical polymer e-caps is, however, that the separator is impregnated with a liquid electrolyte as in a conventional wet aluminum electrolytic capacitor. The liquid electrolyte in operation delivers the oxygen which is necessary for self-healing of the dielectric layer in the presence of any defects.
Such defects in the dielectric are the cause of the higher leakage current values in polymer electrolytic capacitors. Because the current which flows through such a defect, results in a selective heating, which normally destroys the overlying polymer film and the defect is isolated, but not healed. in case of the hybrid polymer capacitors however, through this opening in the polymer layer the liquid electrolyte can pass to the defect, deliver oxygen and heal the dielectric by generating new oxides, whereby the increased leakage current decreases. Due to this self-healing properties hybrid polymer Al capacitors have a much lower leakage current than the polymer Al capacitors.
Comparison of the polymer families
Comparison of benchmarks
The polymer electrolyte, the two different anode materials, aluminum and tantalum, together with the different designs have led to a number of different polymer e-cap families with different benchmarks. An overview over these benchmark values of each family are shown in the table below. For comparison, the basic parameters of the tantalum electrolytic capacitors with manganese dioxide electrolyte are also listed.
| Anode material | Electrolyte | Style | Capacitance range (µF) |
Rated- voltage (V) |
Max. operation- temperature (°C) |
|---|---|---|---|---|---|
| Tantalum | Manganese dioxide | rectangular | 0.1…1,500 | 2.5…63 | 105/125/150/175 |
| Polymer | rectangular | 0.47…3,300 | 2.5…125 | 105/125 | |
| Aluminum | Polymer | rectangular | 2.2…560 | 2.0…16 | 105/125 |
| Polymer | cylindrical (SMD and radial) |
3.3…3,900 | 2.0…200 | 105/125/135 | |
| Hybrid, Polymer and non-solid |
cylindrical (SMD and radial) |
6.8…1,000 | 6.3…125 | 105/125 |
(As of April 2015)
Comparison of electrical parameters
Different electrical properties of different polymer capacitors can best be compared with each other, if they are listed with the same capacitance and rated voltage and in the same dimensions. In such a comparison the values for the ESR and the ripple current are the most important parameters for the use of for polymer capacitors in electronic equipment. Additional the leakage current is listed in this table, because it is higher than that of electrolytic capacitors with other electrolytes than polymer. For a better comparing of the electrical properties of the polymer electrolytic capacitors, the respective values of tantalum electrolytic capacitors with manganese dioxide electrolyte and wet Al e-caps are also listed in the table.
| E-cap family Electrolyte |
Type 1) | Dimensions WxLxH 2) DxL 3) (mm) |
Max. ESR 100 kHz, 20 °C (mΩ) |
Max. Ripple current 85/105 °C (mA) |
Max. Leakage current for 100µF/10V after 2 min. 4) (µA) |
|---|---|---|---|---|---|
| MnO2-Tantalum capacitors MnO2-Electrolyte |
Kemet, T494 330/10 |
7.3x4.3x4.0 | 100 | 1,285 | 10 (0.01CV) |
| MnO2-Tantalum capacitors Multianode, MnO2-Electrolyte |
Kemet, T510 330/10 |
7.3x4.3x4.0 | 35 | 2,500 | 10 (0.01CV) |
| Polymer tantalum capacitors Polymer electrolyte |
Kemet, T543 330/10 |
7.3x4.3x4.0 | 10 | 4,900 | 100 (0.1CV) |
| Polymer tantalum capacitors Multianode, Polymer electrolyte |
Kemet, T530 150/10 |
7.3x4.3x4.0 | 5 | 4,970 | 100 (0.1CV) |
| Polymer aluminum capacitors Polymer electrolyte |
Panasonic, SP-UE 180/6.3 |
7.3x4.3x4.2 | 7 | 3,700 | 40 (0.04CV) |
| Polymer aluminum capacitors Polymer elecrolyte |
Kemet, A700 220/6.3 |
7.3x4.3x4.3 | 10 | 4,700 | 40 (0.04CV) |
| "Wet" aluminum capacitors, SMD Ethylene glycol/Borax-electrolyte |
NIC, NACY, 220/10 |
6.3x8 | 300 | 300 | 10 (0.01CV) |
| "Wet" aluminum capacitors, SMD Water-based electrolyte |
NIC, NAZJ, 220/16 |
6.3x8 | 160 | 600 | 10 (0.01CV) |
| Polymer aluminum capacitors Polymer electrolyte |
Panasonic, SVP 120/6.3 |
6,3x6 | 17 | 2,780 | 200 (0.2CV) |
| Hybrid polymer aluminum capacitors Polymer + non-solid electrolyte |
Panasonic, ZA 100/25 |
6.3x7.7 | 30 | 2,000 | 10 (0.01CV) |
1) Manufacturer, Series, Capacitance/Rated voltage, 2) rectangular style (Chip), 3) cylindrical style, 4) Leakage current, calculated for a capacitor with 100 µF/10 V,
(As of Juni 2015)
Electrical characteristics
Series-equivalent circuit

The electrical characteristics of capacitors are harmonized by the international generic specification IEC 60384-1. In this standard, the electrical characteristics of capacitors are described by an idealized series-equivalent circuit with electrical components which model all ohmic losses, capacitive and inductive parameters of an electrolytic capacitor:
- C, the capacitance of the capacitor
- RESR, the equivalent series resistance which summarizes all ohmic losses of the capacitor, usually abbreviated as "ESR"
- LESL, the equivalent series inductance which is the effective self-inductance of the capacitor, usually abbreviated as "ESL".
- Rleak, the resistance representing the leakage current of the capacitor
Rated capacitance, standard values and tolerances
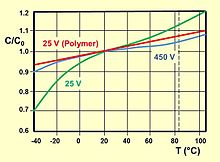
The capacitance value of polymer electrolytic capacitors depends on measuring frequency and temperature. Electrolytic capacitors with non-solid electrolytes show a broader aberration over frequency and temperature ranges than polymer capacitors. .
The standardized measuring condition for polymer Al-e-caps is an AC measuring method with 0.5 V at a frequency of 100/120 Hz and a temperature of 20 °C. For polymer tantalum capacitors a DC bias voltage of 1.1 to 1.5 V for types with a rated voltage ≤2.5 V, or 2.1 to 2.5 V for types with a rated voltage of >2.5 V, may be applied during the measurement to avoid reverse voltage.
The capacitance value measured at the frequency of 1 kHz is about 10% less than the 100/120 Hz value. Therefore, the capacitance values of polymer electrolytic capacitors are not directly comparable and differ from those of film capacitors or ceramic capacitors, whose capacitance is measured at 1 kHz or higher.
The basic unit of a polymer electrolytic capacitor's capacitance is the microfarad (μF). The capacitance value specified in the data sheets of the manufacturers is called the rated capacitance CR or nominal capacitance CN. It is given according to IEC 60063 in values corresponding to the E series. These values are specified with a capacitance tolerance in accordance with IEC 60062, so that no overlaps arise.
| E3-series | E6-series | E12-series |
|---|---|---|
| 10-22-47 | 10-15-22-33-47-68 | 10-12-15-18-22-27 33-39-47-65-68-82 |
| capacitance tolerance ±20% | capacitance tolerance ±20% | capacitance tolerance ±10% |
| letter code "M" | letter code "M" | letter code "K" |
The actual measured capacitance value must be within the tolerance limits.
Rated and category voltage

Referring to the IEC 60384-1 standard, the allowed operating voltage for polymer electrolytic capacitors is called the "rated voltage UR". The rated voltage UR is the maximum DC voltage or peak pulse voltage that may be applied continuously at any temperature within the rated temperature range TR.
The voltage proof of electrolytic capacitors decreases with increasing temperature. For some applications it is important to use a higher temperature range. Lowering the voltage applied at a higher temperature maintains safety margins. For some capacitor types therefore the IEC standard specifies a "temperature derated voltage" for a higher temperature, the "category voltage UC". The category voltage is the maximum DC voltage or peak pulse voltage that may be applied continuously to a capacitor at any temperature within the category temperature range TC. The relation between both voltages and temperatures is given in the picture at right.
Applying a higher voltage than specified may destroy electrolytic capacitors.
Applying a lower voltage may have a positive influence on polymer electrolytic capacitors. For hybrid polymer aluminum electrolytic capacitors a lower applied voltage can in some cases extend the lifetime.[20] For polymer tantalum electrolytic capacitors lowering the voltage applied increases the reliability and reduces the expected failure rate.[50]
Rated and category temperature
The relation between rated temperature TR and rated voltage UR as well as higher category temperature TC and derated category voltage UC is given in the picture at right.
Surge Voltage
Polymer electrolytic capacitors are formed for safety reasons at a higher voltage than only at the rated voltage. Therefore, it is allowed to apply for short times a surge voltage for a limited number of cycles.
The surge voltage indicates the maximum peak voltage value that may be applied to polymer electrolytic capacitors during their application for a limited number of cycles.[20] The surge voltage is standardized in IEC 60384-1.
For polymer aluminum electrolytic capacitors the surge voltage is 1.15 times the rated voltage. For tantalum electrolytic capacitors the surge voltage can be 1.3 times the rated voltage, rounded off to the nearest volt.
The surge voltage applied to polymer capacitors may influence the capacitor's failure rate.[51][52][53]
Transient Voltage
Transients are fast and high voltage spikes. Polymer electrolytic capacitors, aluminum as well as tantalum polymer capacitors can not withstand transients or peak voltages higher than surge voltage. Transients for this type of e-caps may destroy the components.[51][52]
Hybrid polymer aluminum capacitors are relatively insensitive to high and short- term transient voltages higher than surge voltage, if the frequency and the energy content of the transients are low.[17][41] This ability depends on rated voltage and component size. Low energy transient voltages lead to a voltage limitation similar to a zener diode.[54] An unambiguous and general specification of tolerable transients or peak voltages is not possible. In every case transients arise, the application has to be approved very carefully.
Reverse voltage
Polymer electrolytic capacitors, tantalum as well as aluminum polymer capacitors are polarized capacitors and generally requires the anode electrode voltage to be positive relative to the cathode voltage. Nevertheless, polymer electrolytic capacitors can withstand for short instants a type dependent reverse voltage for a limited number of cycles.[55][56] A reverse voltage higher than the type-dependent threshold level applied for a long time to the polymer electrolyte capacitor leads to short-circuit and therefore to the destruction of the capacitor.
To minimize the likelihood of a polarized electrolytic being incorrectly inserted into a circuit, polarity has to be very clearly indicated on the case, see the section on "Polarity marking" below.
Impedance and ESR
See also: Electrolytic capacitor#Impedance and Electrolytic capacitor#ESR and dissipation factor tan δ
The impedance is the complex ratio of the voltage to the current in an AC circuit, and possesses as AC resistance both magnitude and phase at a particular frequency. In the data sheets of polymer electrolyte capacitors only the impedance magnitude |Z| is specified, and simply written as "Z". Regarding the IEC 60384-1 standard, the impedance values of polymer electrolytic capacitors are measured and specified at 100 kHz.
In the special case of resonance, in which the both reactive resistances XC and XL have the same value (XC=XL), then the impedance will only be determined by equivalent series resistance ESR, which summarizes all resistive losses of the capacitor. At the measuring frequency 100 kHz the impedance and the ESR have nearly the same value for polymer capacitors with capacitance values in the µF range. With frequencies above the resonance the impedance increases again due to the ESL of the capacitor. Than the capacitor becomes an inductance.


The impedance and the ESR of polymer electrolytic capacitors, as shown in the curves, heavily depend on the used electrolyte. The curves show the development of the impedance and the ESR towards smaller values from the "wet" Al electrolytic capacitors over tantalum capacitors with MnO2 electrolyte, Al electrolytic capacitors with TCNQ electrolytes to tantalum polymer capacitors. Additional is shown the curve of a ceramic Class 2 MLCC capacitor, which have still lower Z and ESR values, whose capacitance however, is strongly dependent on the voltage.
An advantage of the polymer electrolyte capacitors against Al electrolytic capacitors with liquid electrolyte is the low temperature dependence and the almost linear curve of the ESR over the entire specified temperature range. This applies both to polymer tantalum, polymer aluminum, as well as for hybrid polymer aluminum electrolytic capacitors. The impedance and the ESR of polymer capacitors is also still dependent on the design and materials of the capacitor. Due to their construction have wound capacitors a higher inductance than capacitors with layered electrodes. The rectangular polymer Al and Ta-capacitors with the same capacitance therefore have a higher resonant frequency compared with cylindrical designs. This effect is further amplified by the multi-anode technique, in which the individual inductances are reduced by the parallel circuit[45][46] and the "face-down" technique at polymer Ta capacitors,.[47]
Ripple current

A "ripple current" is the RMS value of a superimposed AC current of any frequency and any waveform of the current curve for continuous operation within the specified temperature range. It arises mainly in power supplies (including switched-mode power supplies) after rectifying an AC voltage and flows as charge and discharge current through the decoupling or smoothing capacitor.[57]
Ripple currents generates heat inside the capacitor body. This dissipation power loss PL is caused by ESR and is the squared value of the effective (RMS) ripple current IR.
This internally generated heat, additional to the ambient temperature and possibly other external heat sources, leads to a capacitor body temperature having a temperature difference of Δ T against the ambient. This heat has to be distributed as thermal losses Pth over the capacitor's surface A and the thermal resistance β to the ambient.
The internally generated heat has to be distributed to the ambient by thermal radiation, convection, and thermal conduction. The temperature of the capacitor, which is the net balance between heat produced and distributed, must not exceed the capacitor's maximum specified temperature.
The ripple current for polymer capacitors is specified as an effective (RMS) value at 100 kHz at upper category temperature. Since the ESR of polymer capacitors is relatively stable over all the frequency range simplified the 100 kHz-value could be used for the entire frequency range. Typically, the specified rated value for maximum ripple current in manufacturers' data sheets of polymer capacitors is calculated for a heating the capacitor core (cell) of 20 °C. Any use of polymer capacitors in the extended area of the category temperature reduces the specified ripple current.
Non-sinusoidal ripple currents have to be analyzed and separated into their single sinusoidal frequencies by means of Fourier analysis and summarized by squared addition the single currents.[58]
In polymer tantalum electrolytic capacitors the heat generated by the ripple current influences the reliability of the capacitors.[59][60][61][62] Exceeding the limit tends to result in catastrophic failures with short circuits and burning components.
The heat generated by the ripple current also influences the lifetime of aluminum and tantalum electrolytic capacitors with solid polymer electrolytes.[63]
For hybrid polymer electrolyte capacitors the ripple current also affects the life expectancy of the capacitors.[57]
Current surge, peak or pulse current
Polymer tantalum electrolytic capacitors are sensitive against peak or pulse currents.[51][52] Solid Tantalum capacitors which are exposed to surge, peak or pulse currents, for example, in highly inductive circuits, should be used with a voltage derating. If possible the voltage profile should be a ramp turn-on, as this reduces the peak current experienced by the capacitor.
Polymer aluminum capacitors don’t have restrictions against current surge, peak or pulse currents. Only the summarized currents must not exceed the maximal specified ripple current.
Leakage current

The DC leakage current (DCL) is a special characteristic for electrolytic capacitors other conventional capacitors don’t have. It is the DC current which flows through the electrolytic capacitor when a DC voltage of correct polarity is applied. This current is represented by the resistor Rleak in parallel with the capacitor in the series-equivalent circuit of electrolytic capacitors. The main causes of DCL for solid polymer capacitors are f. e. points of electrical breakdown of the dielectric after soldering, conductive paths due to impurities or due to poor anodization, and for rectangular types bypassing of dielectric due to excess manganese dioxide, due to moisture paths or due to cathode conductors (carbon, Silver).[64]
The specification of the leakage current in datasheets often will be given by multiplication of the rated capacitance value CR with the value of the rated voltage UR together with an addendum figure, measured after a measuring time of 2 or 5 minutes, for example:
Leakage current in solid polymer electrolytic capacitors generally drops very fast but than remain on the reached level. The value of the leakage current of polymer electrolytic capacitors depends on the voltage applied, on temperature of the capacitor, on measuring time, and on influence of moisture caused by case sealing conditions.
Polymer electrolytic capacitors have relatively high leakage current values. This "normal" leakage current in solid polymer electrolyte capacitors couldn’t be reduced by "healing" in the sense of generating new oxide because under normal conditions solid electrolytes can't deliver oxygen for forming processes. Annealing of defects in the dielectric layer only can be carried out through local overheating and evaporation of the polymer. The leakage current values for polymer electrolyte capacitors are between 0.2 CRUR to 0.04 CRUR, depending on the manufacturer and series. Thus the value of the leakage current for polymer capacitors is higher than for "wet" aluminum electrolytic capacitors and tantalum capacitors with MnO2 electrolyte.
The disadvantage of the higher leakage current of solid polymer capacitors compared with other e-caps families is prevented by design of the hybrid polymer aluminum electrolytic capacitors. In these hybrid capacitors, the liquid electrolyte provides the oxygen which is necessary for the self-healing or reforming of defects in the oxide, so that the leakage current of the hybrid polymer capacitors achieved the same values as in wet Al electrolytic capacitors or tantalum capacitors.[17][57]
Dielectric absorption (soakage)
Dielectric absorption occurs when a capacitor that has remained charged for a long time discharges only incompletely when briefly discharged. Although an ideal capacitor would reach zero volts after discharge, real capacitors develop a small voltage from time-delayed dipole discharging, a phenomenon that is also called dielectric relaxation, "soakage" or "battery action".
For polymer tantalum as well as aluminum electrolytic capacitors no figures for dielectric absorption are available.
Reliability and lifetime
Reliability (failure rate)

The reliability of a component is a property that indicates how reliably this component performs its function in a time interval. It is subject to a stochastic process and can be described qualitatively and quantitatively; it is not directly measurable. The reliability of electrolytic capacitors is empirically determined by identifying the failure rate in production accompanying endurance tests, see Reliability engineering. Reliability normally is shown as a bathtub curve and is divided into three areas: early failures or infant mortality failures, constant random failures and wear out failures. Failures totalized in a failure rate are short circuit, open circuit, and degradation failures (exceeding electrical parameters). For polymer tantalum capacitors the failure rate is also influenced by the circuit series resistor, which is not required for polymer aluminum capacitors.
Billions of tested capacitor unit-hours would be needed to establish failure rates in the very low level range which are required today to ensure the production of large quantities of components without failures. This requires about a million units over a long time period, which means a large staff and considerable financing.[65] The tested failure rates are often complemented with figures resulting from feedback from the field from big users concerning failed components (field failure rate), which mostly results in a lower failure rate than tested.
Out of historical reasons the failure rate units of tantalum and aluminum electrolytic capacitors are different. For aluminum electrolytic capacitors the reliability prediction is generally expressed in a failure rate λ, with the unit FIT (Failures In Time] at fixed standard operating conditions 40 °C and 0.5 UR during the period of constant random failures. This is the number of failures that can be expected in one billion (109) component-hours of operation (e.g., 1000 components for 1 million hours, or 1 million components for 1000 hours which is 1 ppm/1000 hours) at the standard operating conditions. This failure rate model implicitly assumes the idea of "random failure". Individual components fail at random times but at a predictable rate. The reciprocal value of FIT is MTBF (Mean Time Between Failures).
For tantalum capacitors the failure rate "FTa" is specified with the unit "n % failures per 1000 hours" at 85 °C, U = UR and a circuit resistance of 0.1 Ω/V. This is the percentage of failures that can be expected in 1000 hours of operation at this very much higher operational conditions compared with the "FIT" model. The failure rates "λ" and "FTa" are depending on operational conditions like temperature, voltage applied, and various environmental factors such as humidity, shocks or vibrations and of the capacitance value of the capacitor.[50] The higher the temperature and applied voltage the higher the failure rate, for example. For standard solid tantalum and "wet" aluminum electrolytic capacitors both failure rates can be recalculated with acceleration factors standardized for industrial[66] or military[67] contexts. The latter is established in the industry and also often used for industrial applications. However, for polymer aluminum and tantalum electrolytic capacitors up to now no acceleration factors are published. An example of a recalculation from a tantalum capacitor failure rate FTa into a failure rate λ therefore only can be given by comparing standard capacitors. Example:
A failure rate FTa = 0.1%/1000 h at 85 °C and U= UR shall be recalculated into a failure rate λ at 40 °C and U = 0,5 UR.
The following acceleration factors from MIL-HDBK 217F are used:
- FU = voltage acceleration factor, for U = 0,5 UR is FU = 0.1
- FT = temperature acceleration factor, for T = 40 °C is FT = 0.1
- FR = acceleration factor for the series resistance RV, at the same value it is = 1
It follows
- λ = FTa x FU x FT x FR
- λ = (0.001/1000 h) x 0.1 x 0.1 x 1 = 0.00001/1000 h = 1•10−9/h = 1 FIT
As of 2015 the published failure rate figures for polymer tantalum as well as for polymer aluminum capacitors, which come from many manufacturers, are in the range of 0.5 to 20 FIT. That means that these electrolytic capacitors are reliable components, comparable with other electronic components and achieving safe operation for decades under normal conditions, and within the calculated lifeme.
Lifetime, service life
The life time, service life, load life or useful life of electrolytic capacitors is a special characteristic of non-solid electrolytic capacitors, especially non-solid aluminum electrolytic capacitors whose liquid electrolyte can evaporate over the time leading to wear-out failures. Solid tantalum capacitors with manganese dioxide electrolyte have no wear-out mechanism so that the constant failure rate least up to the point all capacitors have failed. They don’t have a life time specification like non-solid aluminum electrolytic capacitors.
However, solid polymer tantalum as well as aluminum electrolytic capacitors do have a life time specification. The polymer electrolyte has a small deterioration of conductivity by a thermal degradation mechanism of the conductive polymer. The electrical conductivity decreased, as a function of time, in agreement with a granular metal type structure, in which aging is due to the shrinking of the conductive polymer grains.[63]
The time of the capacitors functionality (useful life, load life, service life) is tested with a time accelerating test called "endurance test" according to the relevant IEC standards IEC 60384-24/-25/-26[68] with rated voltage at the upper category temperature. After this endurance test the specified parameter limits to pass the test are on the one hand no total failures (short circuit, open circuit) and on the other hand, not to reach degradation failures, a reduction of capacitance by more than 20% and an increase of the ESR, impedance or loss factor by more than a factor of 2, compared to the initial value. Parameters of the tested component beyond these limits can be count as degradation failure. These specified limits for polymer capacitor degradation failures are much closer than for wet aluminum capacitors. That means, the life time behavior of polymer e-caps are much more stable than for wet Al e-caps.
The life time of polymer electrolytic capacitors for max. voltage and max. temperature is specified in similar terms to the non-solid electrolytic caps, but its life time calculation for lower operational conditions follows other rules which lead to much longer operational life times.[69][70][71] The polymer capacitors life time for different operational conditions can be estimated by the formula:
- Lx = life time to be estimated
- LSpec = specified life time (useful life, load life, service life)
- T0 = upper category temperature (°C)
- TA = temperature (°C) of the e-cap case or ambient temperature near the capacitor
This rule characterizes the change of thermic polymer reactions speed within the specified degradation limits. According to this formula the theoretical expected service life of a 2000 h/105 °C polymer capacitor, which is operated at 65 °C, can be calculated (better estimated) with 200,000 hours or more than 20 years.
For hybrid polymer Al electrolytic capacitors, which contain a liquid electrolyte, the 20-degree rule does not apply. The expected life of these hybrid electrolytic capacitors can be calculated using the well-known 10-degree rule, see Aluminum electrolytic capacitor#Lifetime, service life
Failure modes, self-healing mechanism and application rules
Polymer capacitors, tantalum as well as aluminum, are reliable on the same very high level as other electronic components with very low failure rates. However, all tantalum electrolytic capacitors, polymer tantalum too, have a single unique failure mode called "field crystallization".[72]
Field crystallization is the major reason for degradation and catastrophic failures of solid tantalum capacitors.[73] More than 90% of the today's rare failures in tantalum solid-state electrolytic capacitors are caused by shorts or increased leakage current due to this failure mode.[74]
The extremely thin oxide film of a tantalum electrolytic capacitor, the dielectric layer, must be formed in an amorphous structure. Changing the amorphous structure into a crystallized structure the conductivity is reported to be 1000 times higher combined with an enlargement of the oxide volume.[23][75]

The field crystallization followed by an dielectric breakdown is characterized by a sudden rise in leakage current, within a few milliseconds, from nano-ampere magnitude to ampere magnitude in low-impedance circuits. Increasing current flow can be accelerate as an "avalanche effect", and rapidly spread through the metal/oxide. This can result in various degrees of destruction from rather small, burned areas on the oxide to zigzag burned streaks covering large areas of the pellet or complete oxidation of the metal.[76][77] If the current source is unlimited a field crystallization may cause a capacitor short circuit. However, if the current source is limited in tantalum electrolytic capacitors with solid MnO2 electrolyte a self-healing process take place oxidizing MnO2 into insulating Mn2O3
In polymer tantalum capacitors a danger of burning capacitors does not exist. It is true that even in the polymer tantalum capacitors field crystallization occurs, however, in this case, the polymer layer is selectively heated and burned away by the increasing leakage current, so that the faulty point is insulated. Since the polymer material no oxygen can provide the leakage current can’t accelerate. Only the faulty area is isolated by and contributes no longer to the capacitance of the capacitor.
Polymer aluminum capacitors show the same self-healing mechanism like polymer tantalum capacitors. After application of a voltage at weakened spots in the oxide of the capacitor a localized higher leakage current is formed, which leads to a local heating of the polymer, whereby the polymer either oxidized and becomes highly resistive or evaporates. Also hybrid polymer capacitors show this self-healing mechanism. However, if this faulty spot is not covered with a polymer film the liquid electrolyte can reach this position and can deliver oxygen to build up new dielectric oxide. This is the reason for relatively low leakage current values for the hybrid polymer capacitors.
The many different types of polymer electrolytic capacitors show differences in electrical long-term behavior, their inherent failure modes, and their self-healing mechanism. To prevent sudden failures, manufacturers recommend different application rules, see following table:
| Type of electrolytic capacitors |
Long-term electrical behavior |
Failure modes | Self-healing mechanism |
Application rules |
|---|---|---|---|---|
| "Wet" aluminum electrolytic capacitors, |
Drying out over time, capacitance decreases, ESR increases |
no unique determinable |
New generated oxide (forming) by applying a voltage |
Lifetime calculation 10 °C rule |
| Polymer aluminum electrolytic capacitors |
Deterioration of conductivity, ESR increases |
no unique determinable |
Insulating of faults in the dielectric by oxidation or evaporation of the polymer electrolyte |
Lifetime calculation 20 °C rule |
| MnO2 tantalum electrolytic capacitors, |
Stable | Field crystallization [23][76] |
Thermally induced insulating of faults in the dielectric by oxidization of the electrolyte MnO2 into insulating MnO2O3 if current availability is limited |
Voltage derating 50% Series resistance 3 Ω/V [77][78] |
| Polymer tantalum electrolytic capacitors |
Deterioration of conductivity, ESR increases |
Field crystallization [23][76] |
Insulating of faults in the dielectric by oxidation or evaporation of the polymer electrolyte |
Voltage derating 20 % [77][78] |
| Hybrid polymer aluminum electrolytic capacitors |
Deterioration of conductivity, drying out over time, capacitance decreases, ESR increases |
no unique determinable |
New generated oxide (forming) by applying a voltage |
Lifetime calculation 10 °C rule |
Additional informations
Capacitor symbol
Electrolytic capacitor symbols
| Electrolytic capacitor |
Electrolytic capacitor |
Electrolytic capacitor |
Polarity marking
Polarity marking for polymer electrolytic capacitors
 |
 |
| Rectangular polymer capacitors, tantalum as well as aluminum, have a polarity marking at the anode (plus) side |
Cylindrical polymer capacitors |
Imprinted markings
Polymer electrolytic capacitors, if enough space is available, have coded imprinted markings to indicate:
- manufacturer's name or trademark;
- manufacturer's type designation;
- polarity
- rated capacitance;
- tolerance on rated capacitance
- rated voltage
- climatic category or rated temperature;
- year and month (or week) of manufacture;
For very small capacitors no marking is possible.
The code of the imprinted markings differ from manufacturer to manufacturer.
Standardization
The standardization for all electronic components and related technologies follows the rules given by the International Electrotechnical Commission (IEC),[79] a non-profit, non-governmental international standards organization.[80][81]
The definition of the characteristics and the procedure of the test methods for capacitors for use in electronic equipment are set out in the Generic specification:
- IEC/EN 60384-1 - Fixed capacitors for use in electronic equipment
The tests and requirements to be met by aluminum and tantalum electrolytic capacitors for use in electronic equipment for approval as standardized types are set out in the following sectional specifications:
- IEC/EN 60384-24—Surface mount fixed tantalum electrolytic capacitors with conductive polymer solid electrolyte
- IEC/EN 60384-25—Surface mount fixed aluminium electrolytic capacitors with conductive polymer solid electrolyte
- IEC/EN 60384-26—Fixed aluminium electrolytic capacitors with conductive polymer solid electrolyte
Advantages and disadvantages
Advantages of polymer e-caps against wet e-caps:
- articulately lower ESR values.
- articulately higher ripple current capability
- articulately lower temperature depending characteristics
- no evaporation of electrolyte, longer service life
- no burning or exploding in case of shorts
Disadvantages of polymer e-caps against wet e-caps:
- more expensive
- higher leakage current
- damageable by transients and higher voltages spikes
Advantages of hybrid polymer aluminum e-caps:
- less expensive than polymer aluminum e-caps
- lower leakage current
- impassible against transients
Disadvantage of hybrid polymer e-caps:
- limited service life due to evaporation
Advantages of polymer e-caps against MLCCs:
- no voltage dependent capacitance
- no microphonic
- higher capacitance values possible
Technological competition
The ESR and ESL characteristics of polymer electrolytic capacitors on the one hand side are increasingly converging to those of MLCC capacitors. On the other hand, the specific capacitance of Class 2-MLCC capacitors approaches more and more to that of tantalum chip capacitors.[82][83] However, apart from this increasing comparability there are arguments in favor of or against certain types of capacitors. Many capacitor manufacturers compose these crucial arguments of their technologies against the competition in presentations and articles.[84][85] Here is a small selection of special comparisons:
- Al-Polymer e-caps against MLCC: Panasonic[86]
- MLCC against Polymer and "wet" e-caps:Murata[87][88]
- Al-Polymer e-caps against "wet" e-caps: NCC[18] NIC[1]
- Ta-Polymer e-caps against standard solid Ta-MnO2 e-caps: Kemet[89]
Manufacturers and products
| Manufacturer | Polymer Tantalum capacitors |
Polymer Aluminum capacitors | ||
|---|---|---|---|---|
| rectangular SMD |
rectangular SMD |
cylindric leaded SMD, V-Chip |
cylindric Hybrid | |
| AVX | X | - | - | - |
| AISHI | - | X | X | - |
| CapXon | - | - | X | - |
| CDE Cornell Dubilier | X | - | - | X |
| Elite | - | - | X | - |
| Elna | - | - | X | - |
| Illinois | - | X | X | - |
| Jianghai | - | - | X | - |
| KEMET | X | X | - | - |
| Lelon | - | - | X | - |
| Matsuo | X | X | - | - |
| Murata | - | X | - | - |
| Nippon Chemi-Con | - | - | X | X |
| NIC | X | - | X | X |
| Nichicon | - | X | X | - |
| Panasonic | X | X | X | X |
| PolyCap | - | - | X | - |
| ROHM | X | - | - | - |
| Rubycon | - | X | - | - |
| Samsung | X | - | - | - |
| Samwha | - | - | - | X |
| Sun Electronic (Suncon) | - | - | - | X |
| Teapo/Luxon | - | - | X | - |
| Vishay | X | - | - | - |
| Yageo | - | - | X | |
As of July 2015
See also
- Aluminum electrolytic capacitor
- Electrolytic capacitor
- Niobium capacitor
- SAL electrolytic capacitor
- Tantalum capacitor
- Capacitor types
References
- ^ a b c NIC Components Corp., Hybrid Construction, Aluminum Electrolytic Capacitors [1]
- ^ R. L. Taylor and H. E. Haring, "A metal semi-conductor capacitor," J. Electrochem. Soc., vol. 103, S. 611, November, 1956.
- ^ D. A. McLean, F. S. Power, Proc. Inst. Radio Engrs. 44 (1956) 872
- ^ a b Capacitor Impedance Needs For Future Microprocessors, Larry E. Mosley, Intel Corporation, CARTS USA 2006 April 3–6, 2006 Orlando, FL, [2]
- ^ F. Wudl, "From organic metals to superconductors: managing conduction electrons in organic solids", In: Accounts of Chemical Research. 17, Nr. 6, 1984, S. 227–232, doi:10.1021/ar00102a005.
- ^ Shinichi Niwa, Yutaka Taketani, Development of new series of aluminium solid capacitors with organic Semiconductive electrolyte (OS-CON), Journal of Power Sources, Volume 60, Issue 2, June 1996, Pages 165–171, [3]
- ^ Kuch, Investigation of charge transfer complexes:TCNQ-TTF
- ^ Sanyo, OS-CON, Technical Book Ver. 15, 2007
- ^ About the Nobel Prize in Chemistry 2000, Advanced Information, October 10, 2000,[4]
- ^ Y. K. Zhang, J. Lin,Y. Chen, Polymer Aluminum Electrolytic Capacitors with Chemically-Polymerized Polypyrrole (PPy) as Cathode Materials Part I. Effect of Monomer Concentration and Oxidant on Electrical Properties of the Capacitors, PDF
- ^ U. Merker, K. Wussow, W. Lövenich, H. C. Starck GmbH, New Conducting Polymer Dispersions for Solid Electrolyte Capacitors, PDF
- ^ APYCAP Series, Function Polymer Capacitor, Nitsuko, Datasheet 1988
- ^ "Electronic Components - Panasonic Industrial Devices". panasonic.com. Retrieved 22 October 2015.
- ^ John Prymak, Kemet, Replacing MnO2 with Polymers, 1999 CARTS
- ^ F. Jonas, H.C. Starck, Baytron, Basic chemical and physical properties, Präsentation 2003, [www.hcstarck.de]
- ^ John Prymak, Kemet, "Performance Improvements with Polymer (Ta and Al)", 2001 APEC [5]
- ^ a b c d "Understanding Polymer & Hybrid Capacitors [Whitepaper] - Panasonic Industrial Devices". panasonic.com. Retrieved 22 October 2015.
- ^ a b Nippon Chemi-Con, Conductive Polymer Aluminum Solid Capacitors Application Note PDF
- ^ J.L. Stevens, A.C. Geiculescu, T.F. Strange, Dielectric Aluminum Oxides: Nano-Structural Features and Composites PDF
- ^ a b c A. Albertsen, Jianghai Europe, Keep your distance – Voltage Proof of Electrolytic Capacitors, PDF
- ^ KDK, Specifications for Etched Foil for Anode, Low Voltage
- ^ I. Horacek, T. Zednicek, S. Zednicek, T. Karnik, J. Petrzilek, P. Jacisko, P. Gregorova, AVX, High CV Tantalum Capacitors - Challenges and Limitations [6]
- ^ a b c d T. Zednicek, AVX, A Study of Field Crystallization in Tantalum Capacitors and its effect on DCL and Reliability, [7]
- ^ Panasonic Announces that it Makes SANYO its Wholly-owned Subsidiary through Share Exchange PDF
- ^ "Electronic Components - Panasonic Industrial Devices" (PDF). panasonic.com. Retrieved 22 October 2015.
- ^ Wolfgang Kaiser, Kunststoffchemie für Ingenieure, 3. Auflage, Carl Hanser, München, 2011, ISBN 978-3-446-43047-1 [8]
- ^ "Elektrisch leitfähige Polymere". chemgapedia.de. Retrieved 22 October 2015.
- ^ a b "Elektrisch leitfähige Polymere". chemgapedia.de. Retrieved 22 October 2015.
- ^ a b c d e f A. Elschner, St. Kirchmeyer, W. Lövenich, U. Merker, K. Reuter, H.C. Starck GmbH, “PEDOT Principles and Applications of an Intrinsically Conductive Polymer”, CRC Press, Taylor and Francis Group, LLC, 2011, November 2, 2010, ISBN 978-1-4200-6911-2, [9]
- ^ "Polypyrrole: a conducting polymer; its synthesis, properties and applications" Russ. Chem. Rev. 1997, vol. 66, p.443ff, [10]
- ^ S. Machida, S. Miyata, A. Techagumpuch: Chemical synthesis of highly electrically conductive polypyrrole. In: Synthetic Metals. 31, Nr. 3, 1989, S. 311–318,doi:10.1016/0379-6779(89)90798-4
- ^ Masashi Oshima, Rubycon, Conductive Polymer Aluminum for Electrolytic Capacitor Technology [11]
- ^ Rubycon, Conductive Polymer Aluminum Solid Electrolytic Capacitors "PZ-CAP" Introduction PDF
- ^ a b U. Merker, K. Reuter, K. Wussow, S. Kirchmeyer, and U. Tracht, „PEDT as conductive polymer cathode in electrolytic capacitors”. CARTS Europe 2002
- ^ "Conductive Polymers". montana.edu. Retrieved 22 October 2015.
- ^ a b "Clevios™ Solid Electrolyte Capacitors". heraeus-clevios.com. Retrieved 22 October 2015.
- ^ Correlation of morphology and charge transport in poly(3,4-ethylenedioxythiophene)–Polystyrenesulfonic acid (PEDOT–PSS) films, C.S. Suchand Sangeeth, Manu Jaiswal, Reghu Menon, Department of Physics, Indian Institute of Science, Bangalore 560012, India, [12]
- ^ A. M. Nardes: On the conductivity of PEDOT:PSS thin films. Doctoral degree 18-12-2007. doi:10.6100/IR631615 http://repository.tue.nl/631615 [13]
- ^ a b A. Albertsen, Jianghai, polymer aluminum electrolytic capacitors with 200 V dielectric strength, elektroniknet.de, 10.17.2014 [14]
- ^ Zhaoqing Beryl Electronic Technology Co., Ltd, 250 V Polymer capacitor series CB, [15]
- ^ a b NIC Components Corp., Hybrid Construction, Aluminum Electrolytic Capacitors PDF
- ^ "Tantalum capacitor powder product information - H.C. Starck". hcstarck.com. Retrieved 22 October 2015.
- ^ H. Haas, H. C. Starck GmbH, Magnesium Vapour Reduced Tantalum Powders with Very High Capacitances [16]
- ^ J. Gill, AVX, Basic Tantalum Capacitor Technology, [17] or [18]
- ^ a b Reed/Marshall, Kemet, "Stable, Low-ESR Tantalum Capacitors", 2000 CARTS [19]
- ^ a b T. Zedníček, L. Marek, S. Zedníček, AVX, New Low Profile Low ESL Multi-Anode "Mirror" Tantalum Capacitor, [20]
- ^ a b E. Chen, K. Lai, J. Prymak. M. Prevallet, Kemet, "Facedown Termination for Higher C/V – Lower ESL Conductive-Polymer SMT Capacitors", CARTS Asia, October 2005 [21]
- ^ Pansonic, Al-Polymer-e-caps, series TPC, 330 µF, 6,3 V, 7,3x4,3x1,9 mm, ESR=40 mΩ, rippel current=1900 mA is comparable with Kemet, Ta-Polymer-e-cap, series T545, 330 µF, 6,3 V, 7,3x4,3x2,0 mm, ESR=45 mΩ, rippel current=2000 mA
- ^ Nichicon, series CG, 4700 µF/2,5 V, 10x12,7 mm, ESR=8 mΩ, ripple current=7 A (105 °C, 100 kHz), [22]
- ^ a b Ch. Reynolds, AVX, Technical Information, Reliability Management of Tantalum Capacitors, PDF
- ^ a b c J. Gill, AVX, Surge in Solid Tantalum Capacitors
- ^ a b c A. Teverovsky, Perot Systems Code 562, NASA GSFCE, Effect of Surge Current Testing on Reliability of Solid Tantalum Capacitors PDF
- ^ D. Liu, M. J. Sampson, NASA Goddard Space Flight Center, Physical and Electrical Characterization of Aluminum Polymer Capacitors, [23]
- ^ Imam, A.M., Condition Monitoring of Electrolytic Capacitors for Power Electronics Applications, Dissertation, Georgia Institute of Technology (2007) PDF
- ^ I. Bishop, J. Gill, AVX Ltd., Reverse Voltage Behavior of Solid Tantalum Capacitors PDF
- ^ P. Vasina, T. Zednicek, Z. Sita, J. Sikula, J. Pavelka, AVX, Thermal and Electrical Breakdown Versus Reliability of Ta2O5 Under Both – Bipolar Biasing Conditions PDF
- ^ a b c Nippon Chemi-Con, Conductive Polymer Aluminum Solid Capacitors, Application Note, 2009.7. Rev. 03 [24]
- ^ Vishay BCcomponents, Introduction Aluminum Capacitors, Revision: 10-Sep-13 1 Document Number: 28356, PDF
- ^ I. Salisbury, AVX, Thermal Management of Surface Mounted Tantalum Capacitors [25]
- ^ R.W. Franklin, AVX, Ripple Rating of Tantalum Chip Capacitors
- ^ Vishay, Application Notes, AC Ripple Current, Calculations Solid Tantalum Capacitors [26]
- ^ KEMET, Ripple Current Capabilities, Technical Update 2004
- ^ a b E. Vitoratos, S. Sakkopoulos, E. Dalas, N. Paliatsas, D. Karageorgopoulos, F. Petraki, S. Kennou, S.A. Choulis, Thermal degradation mechanisms of PEDOT:PSS, Organic Electronics Volume 10, Issue 1, February 2009, Pages 61–66, Organic ElectronicsVolume 10, Issue 1, February 2009, Pages 61–66, [27]
- ^ R.W. Franklin, AVX, An Exploration of Leakage Current
- ^ NIC, Failure Rate Estimation
- ^ IEC/EN 61709, Electric components. Reliability. Reference conditions for failure rates and stress models for conversion
- ^ "MIL-HDBK-217 F NOTICE-2 RELIABILITY PREDICTION ELECTRONIC". everyspec.com. Retrieved 22 October 2015.
- ^ International Electrotechnical Commission [www.iec.ch] or Beuth Verlag [28]
- ^ Nichicon, Technical Guide, Calculation Formula of Lifetime PDF
- ^ Estimating of Lifetime Fujitsu Media Devices Limited PDF
- ^ NIC Technical Guide, Calculation Formula of Lifetime
- ^ B. Goudswaard, F. J. J. Driesens, Failure Mechanism of Solid Tantalum Capacitors, Philips, Electrocomponent Science and Technology, 1976, Vol. 3. pp 171-179 [29]
- ^ Y. Pozdeev-Freeman, Vishay, How Far Can We Go with High CV Tantalum Capacitors, PCI, January/February 2005, page 6, PDF
- ^ Elna, Failure Rates of Tantalum Chip Capacitors
- ^ D. Liu, MEI Technologies, Inc. NASA Goddard Space Flight Center, Failure Modes in Capacitors When Tested Under a Time-Varying Stress [30]
- ^ a b c Vishay, DC Leakage Failure Mode, PDF
- ^ a b c J. Gill, T. Zednicek, AVX, Voltage Derating Rules for Solid Tantalum and Niobium Capacitors, [31]
- ^ a b R. Faltus, AVX, Advanced capacitors ensure long-term control-circuit stability, 7/2/2012, EDT [32]
- ^ IEC - International Electrotechnical Commission. "Welcome to the IEC - International Electrotechnical Commission". iec.ch. Retrieved 22 October 2015.
- ^ IEC Webstore
- ^ "Beuth Verlag - Normen und Fachliteratur seit 1924". beuth.de. Retrieved 22 October 2015.
- ^ R. Hahn, M. Randall, J. Paulson, Kemet, The Battle for Maximum Volumetric Efficiency – Part 1:When Techniques Compete, Customers Win [33]
- ^ R. Hahn, M. Randall, J. Paulson, Kemet, The Battle for Maximum Volumetric Efficiency – Part 2: Advancements in Solid Electrolyte Capacitors [34]
- ^ How do I choose between a polymer aluminum, ceramic and tantalum capacitor? SMT Capacitor Comparison: Polymer Aluminum Chips, Ceramic Chips (X7R, X5R, Z5U, Y5V) and Tantalum Chips, Kemet, [35]
- ^ AN-1099 Application Note, Capacitor Selection Guidelines for Analog Devices, Inc., LDOs, Glenn Morita, PDF
- ^ Specialty Polymer Aluminum Electrolytic Capacitor (SP-AL), Comparison with Multi-Layer Ceramic Capacitor(MLCC) PDF
- ^ Murata Manufacturing Co.,Ltd., TA/AL Cap Replacement PDF
- ^ Murata, Website FAQ April 2010, Polymer Aluminum Electrolytic Capacitors, PDF
- ^ Replacing MnO2 with conductive Polymer in Solid Tantalum Capacitors, John D. Prymak, KEMET Electronics Corp. PDF

















