Ruthenium red
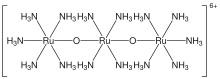
| |
| Identifiers | |
|---|---|
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.228.922 |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| Cl6H42N14O2Ru3 | |
| Molar mass | 786.34 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
The inorganic dye ammoniated ruthenium oxychloride, also known as ruthenium red, is used in histology to stain aldehyde fixed mucopolysaccharides.
Ruthenium red (RR) has also been used as a pharmacological tool to study specific cellular mechanisms. Selectivity is a significant issue in such studies as RR is known to interact with a large number of proteins.[1] These include mammalian ion channels (CatSper1, TASK, RyR1, RyR2, RyR3, TRPM6, TRPM8, TRPV1, TRPV2, TRPV3, TRPV4, TRPV5, TRPV6, TRPA1, mCa1, mCa2, CALHM1[2][3]) TRPP3,[4] a plant ion channel, Ca2+-ATPase, mitochondrial Ca2+ uniporter,[5] tubulin, myosin light-chain phosphatase, and Ca2+ binding proteins such as calmodulin. Ruthenium red displays nanomolar potency against several of its binding partners (e.g. TRPV4, ryanodine receptors,...). For example, it is a potent inhibitor of intracellular calcium release by ryanodine receptors (Kd ~20 nM).[6] As a TRPA1 blocker, it assists in reducing the airway inflammation caused by pepper spray.
RR has been used on plant material since 1890 for staining pectins, mucilages, and gums. RR is a stereoselective stain for pectic acid, insofar as the staining site occurs between each monomer unit and the next adjacent neighbor.[7]
References
- ^ Vincent, F. and Duncton, M. A. J. "TRPV4 Agonists and Antagonists". Current Topics in Medicinal Chemistry 2011; PMID 21671873
- ^ Ma Z, Siebert AP, Cheung KH, Lee RJ, Johnson B, Cohen AS, Vingtdeux V, Marambaud P, Foskett JK. "Calcium homeostasis modulator 1 (CALHM1) is the pore-forming subunit of an ion channel that mediates extracellular Ca2+ regulation of neuronal excitability". Proc Natl Acad Sci USA 2012; 109(28):E1963-71. PMID 22711817
- ^ Dreses-Werringloer U, Vingtdeux V, Zhao H, Chandakkar P, Davies P, Marambaud P. "CALHM1 controls Ca2+-dependent MEK/ERK/RSK/MSK signaling in neurons". J Cell Sci 2013. PMID 23345406
- ^ Decaen, P. G.; Delling, M.; Vien, T. N.; Clapham, D. E. (2013). "Direct recording and molecular identification of the calcium channel of primary cilia". Nature. 504 (7479): 315–318. doi:10.1038/nature12832. PMID 24336289.
- ^ Hajnóczky G, Csordás G, Das S, Garcia-Perez C, Saotome M, Sinha Roy S, Yi M. "Mitochondrial calcium signalling and cell death: approaches for assessing the role of mitochondrial Ca2+ uptake in apoptosis". Cell Calcium 2006;40(5-6):553-60
- ^ Tripathy, Le Xu Ashutosh; Pasek, Daniel A.; Meissner, Gerhard. "Potential for Pharmacology of Ryanodine Receptor/Calcium Release Channels" Ann NY Acad Sci 1998; 853: 130-148 Abstract
- ^ Mariani Colombo P, Rascio N. "Ruthenium red staining for electron microscopy of plant material". Journal of Ultrastructure Research Volume 60, Issue 2, August 1977, Pages 135–139
