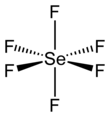
Selenium hexafluoride
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
selenium hexafluoride,
| |||
| Other names
selenium(VI) fluoride, selenium fluoride
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ECHA InfoCard | 100.149.506 | ||
PubChem CID
|
|||
| RTECS number |
| ||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| SeF6 | |||
| Molar mass | 192.9534 g/mol | ||
| Appearance | colourless gas | ||
| Density | 7.887 g/cm3[1] | ||
| Melting point | −34.6 °C [1] | ||
| Boiling point | −46.6 °C, sublimes | ||
| insoluble | |||
Refractive index (nD)
|
1.895 | ||
| Structure | |||
| Orthorhombic, oP28 | |||
| Pnma, No. 62 | |||
| octahedral (Oh) | |||
| 0 | |||
| Thermochemistry | |||
Std enthalpy of
formation (ΔfH⦵298) |
1030 kJ/mol[2] | ||
| Hazards | |||
| NFPA 704 (fire diamond) | |||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Selenium hexafluoride is the inorganic compound with the formula SeF6. It is a colourless gas described as having a "repulsive" odor.[3] It is not widely encountered and has no commercial applications.[4]
Structure, preparation, and reactions
Like many compounds of selenium, SeF6 is hypervalent. The compound has octahedral molecular geometry with Se-F bond length 168.8 pm.
SeF6 can be prepared from the elements[5] or by the reaction of bromine trifluoride (BrF3) with selenium dioxide. The crude product is purified by sublimation.
The relative reactivity of the hexafluorides of S, Se, and Te follows the order TeF6 > SeF6 > SF6, the latter being completely inert toward hydrolysis until high temperatures. SeF6 also resists hydrolysis,[2] The gas can be passed through 10% NaOH or KOH without change, but reacts with gaseous ammonia at 200 °C.[6]
Safety
Selenium hexafluoride is toxic even at low concentrations.[7] In the U.S., OSHA and ACGIH standards for selenium hexafluoride exposure is an upper limit of 0.05 ppm in air averaged over an eight-hour work shift.
References
- ^ a b Lide, D. R., ed. (2005). CRC Handbook of Chemistry and Physics (86th ed.). Boca Raton (FL): CRC Press. ISBN 0-8493-0486-5.
- ^ a b Inorganic Chemistry,Egon Wiberg, Arnold Frederick Holleman Elsevier 2001 ISBN 0-12-352651-5
- ^ "Material Safety" (PDF). Retrieved July 24, 2010.
- ^ Bernd E. Langner "Selenium and Selenium Compounds" in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH, 2002, Weinheim doi:10.1002/14356007.a23_525
- ^ Yost, Don M.; Simons, J. H. (1939). "Sulfur, Selenium, and Tellurium Hexafluorides". 1: 121–122. doi:10.1002/9780470132326.ch44.
{{cite journal}}: Cite journal requires|journal=(help) - ^ Krebs B, Bonmann S, Eidenschink I. Selenium-Inorganic Chemistry Encyclopedia of Inorganic Chemistry Ed. R. Bruce King (1994) John wiley & Sons ISBN 0-471-93620-0
- ^ "Medical Management Guidelines for Selenium Hexafluoride (SeF6)". Retrieved July 24, 2010.