Shikimate pathway
The shikimate pathway (shikimic acid pathway) is a seven step metabolic route used by bacteria, archaea, fungi, algae, some protozoan, and plants for the biosynthesis of folates and aromatic amino acids (phenylalanine, tyrosine, and tryptophan). This pathway is not found in animals, which require these amino acids, hence the products of this pathway represent essential amino acids that must be obtained from organisms that are not animals (or animals which eat those organisms) in the animal's diet.
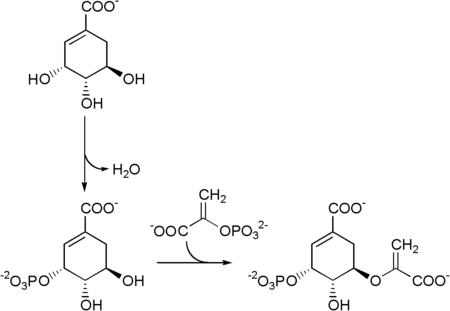
The seven enzymes involved in the shikimate pathway are DAHP synthase, 3-dehydroquinate synthase, 3-dehydroquinate dehydratase, shikimate dehydrogenase, shikimate kinase, EPSP synthase, and chorismate synthase. The pathway starts with two substrates, phosphoenol pyruvate and erythrose-4-phosphate and ends with chorismate, a substrate for the three aromatic amino acids. The fifth enzyme involved is the shikimate kinase, an enzyme that catalyzes the ATP-dependent phosphorylation of shikimate to form shikimate 3-phosphate (shown in the figure below).[1] Shikimate 3-phosphate is then coupled with phosphoenol pyruvate to give 5-enolpyruvylshikimate-3-phosphate via the enzyme 5-enolpyruvylshikimate-3-phosphate (EPSP) synthase.
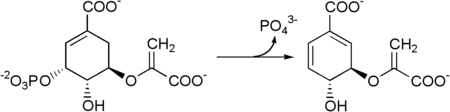
Then 5-enolpyruvylshikimate-3-phosphate is transformed into chorismate by a chorismate synthase.
Prephenic acid is then synthesized by a Claisen rearrangement of chorismate by Chorismate mutase.[2][3]
Prephenate is oxidatively decarboxylated with retention of the hydroxyl group to give p-hydroxyphenylpyruvate, which is transaminated using glutamate as the nitrogen source to give tyrosine and α-ketoglutarate.
References
- ^ Herrmann, K. M.; Weaver, L. M. (1999). "The Shikimate Pathway". Annual Review of Plant Physiology and Plant Molecular Biology. 50: 473–503. doi:10.1146/annurev.arplant.50.1.473. PMID 15012217.
- ^ Helmut Goerisch (1978). "On the mechanism of the chorismate mutase reaction". Biochemistry. 17 (18): 3700. doi:10.1021/bi00611a004.
- ^ Peter Kast, Yadu B. Tewari, Olaf Wiest, Donald Hilvert, Kendall N. Houk, and Robert N. Goldberg (1997). "Thermodynamics of the Conversion of Chorismate to Prephenate: Experimental Results and Theoretical Predictions". J. Phys. Chem. B. 101 (50): 10976–10982. doi:10.1021/jp972501l.
{{cite journal}}: CS1 maint: multiple names: authors list (link)
Bibliography
- Edwin Haslam (1993). Shikimic Acid: Metabolism and Metabolites (1st Edition).
- Brown, Stewart A.; Neish, A. C. (1955). "Shikimic Acid as a Precursor in Lignin Biosynthesis". Nature. 175 (4459): 688–689. doi:10.1038/175688a0. ISSN 0028-0836.
- Weinstein, L. H.; Porter, C. A.; Laurencot, H. J. (1962). "Role of the Shikimic Acid Pathway in the Formation of Tryptophan in Higher Plants : Evidence for an Alternative Pathway in the Bean". Nature. 194 (4824): 205–206. doi:10.1038/194205a0. ISSN 0028-0836.
- Wilson, D J; Patton, S; Florova, G; Hale, V; Reynolds, K A (1998). "The shikimic acid pathway and polyketide biosynthesis". Journal of Industrial Microbiology and Biotechnology. 20 (5): 299–303. doi:10.1038/sj.jim.2900527. ISSN 1367-5435.