Supercritical carbon dioxide
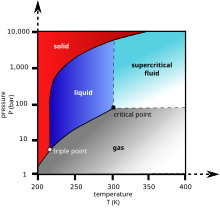
Supercritical carbon dioxide (sCO
2) is a fluid state of carbon dioxide where it is held at or above its critical temperature and critical pressure.
Carbon dioxide usually behaves as a gas in air at standard temperature and pressure (STP), or as a solid called dry ice when frozen. If the temperature and pressure are both increased from STP to be at or above the critical point for carbon dioxide, it can adopt properties midway between a gas and a liquid. More specifically, it behaves as a supercritical fluid above its critical temperature (304.25 K, 31.10 °C, 87.98 °F) and critical pressure (72.9 atm, 7.39 MPa, 1,071 psi), expanding to fill its container like a gas but with a density like that of a liquid.
Supercritical CO
2 is becoming an important commercial and industrial solvent due to its role in chemical extraction in addition to its low toxicity and environmental impact. The relatively low temperature of the process and the stability of CO
2 also allows most compounds to be extracted with little damage or denaturing. In addition, the solubility of many extracted compounds in CO
2 varies with pressure,[1] permitting selective extractions.
Applications
Solvent
Carbon dioxide is gaining popularity among coffee manufacturers looking to move away from classic decaffeinating solvents, because of real or perceived dangers related to their use in food preparation. sCO
2 is forced through the green coffee beans which are then sprayed with water at high pressure to remove the caffeine. The caffeine can then be isolated for resale (e.g. to the pharmaceutical or beverage manufacturers) by passing the water through activated charcoal filters or by distillation, crystallization or reverse osmosis. Supercritical carbon dioxide is used to remove organochloride pesticides and metals from agricultural crops without adulterating the desired constituents from the plant matter in the herbal supplement industry.[2]
Supercritical carbon dioxide can be used as a more environmentally friendly solvent for dry cleaning over traditional solvents such as hydrocarbons, including perchloroethylene.[3]
Supercritical carbon dioxide is used as the extraction solvent for creation of essential oils and other herbal distillates. Its main advantages over solvents such as hexane and acetone in this process are that it is non-toxic and non-flammable. Furthermore, separation of the reaction components from the starting material is much simpler than with traditional organic solvents. The CO
2 can evaporate into the air or be recycled by condensation into a cold recovery vessel. Its advantage over steam distillation is that it operates at a lower temperature, which can separate the plant waxes from the oils.[4]
In laboratories, sCO
2 is used as an extraction solvent, for example for determining total recoverable hydrocarbons from soils, sediments, fly-ash and other media,[5] and determination of polycyclic aromatic hydrocarbons in soil and solid wastes.[6] Supercritical fluid extraction has been used in determining hydrocarbon components in water.[7]
Processes that use sCO
2 to produce micro and nano scale particles, often for pharmaceutical uses, are under development. The gas antisolvent process, rapid expansion of supercritical solutions and supercritical antisolvent precipitation (as well as several related methods) process a variety of substances into particles.[8]
Due to its ability to selectively dissolve organic compounds and assist the functioning of enzymes, sCO
2 has been suggested as a potential solvent to support biological activity on Venus- or super-Earth-type planets.[9]
Manufactured products
Environmentally beneficial, low-cost substitutes for rigid thermoplastic and fired ceramic are made using sCO
2 as a chemical reagent. The sCO
2 in these processes is reacted with the alkaline components of fully hardened hydraulic cement or gypsum plaster to form various carbonates.[10] The primary byproduct is water.
Supercritical carbon dioxide is used in the foaming of polymers. Supercritical carbon dioxide can saturate the polymer with solvent. Upon depressurization and heating the carbon dioxide rapidly expands, causing voids within the polymer matrix, i.e., creating a foam. Research is also ongoing at many universities in the production of microcellular foams using sCO
2.
An electrochemical carboxylation of a para-isobutylbenzyl chloride to ibuprofen is promoted under sCO
2.[11]
Working fluid
Supercritical CO
2 is chemically stable, reliable, low-cost, non-toxic, non-flammable and readily available, making it a desirable candidate working fluid.
Power Generation
The unique properties of sCO
2 present advantages for closed-loop power generation and can be applied to various power generation applications. Power generation systems that use traditional steam Brayton and Rankine cycles can be upgraded to sCO
2 to increase efficiency and power output.
It presents interesting properties that promise substantial improvements in system efficiency. Due to its high fluid density, sCO
2 enables extremely compact and highly efficient turbomachinery. It can use simpler, single casing body designs while steam turbines require multiple turbine stages and associated casings, as well as additional inlet and outlet piping. The high density allows for highly compact, microchannel-based heat exchanger technology.[12]
In 2016, General Electric announced an sCO
2-based turbine that operated at 50% efficiency. In it the CO
2 is heated to 700 °C.It requires less compression and allows heat transfer. It reaches full power in 2 minutes, whereas steam turbines need at least 30 minutes. The prototype generated 10 MW and is approximately 10% the size of a comparable steam turbine.[13]
Further, due to its superior thermal stability and non-flammability, direct heat exchange from high temperature sources is possible, permitting higher working fluid temperatures and therefore higher cycle efficiency. And unlike two-phase flow, sCO
2’s single-phase nature eliminates the necessity of a heat input for phase change that is required for the water to steam conversion, thereby also eliminating associated thermal fatigue and corrosion.[14]
Despite the promise of substantially higher efficiency and lower capital costs, the use of sCO
2 presents material selection and design issues. Materials in power generation components must display high-temperature resistance, oxidation resistance and creep resistance. Candidate materials that meet these property and performance goals include incumbent alloys in power generation, such as nickel-based superalloys for turbomachinery components and austenitic stainless steels for piping. Components within sCO
2 Brayton loops suffer from corrosion and erosion, specifically erosion in turbomachinery and recuperative heat exchanger components and intergranular corrosion and pitting in the piping.[15]
Testing has been conducted on candidate Ni-based alloys, austenitic steels, ferritic steels and ceramics for corrosion resistance in sCO
2 cycles. The interest in these materials derive from their formation of protective surface oxide layers in the presence of carbon dioxide, however in most cases further evaluation of the reaction mechanics and corrosion/erosion kinetics and mechanisms is required, as none of the materials meet the necessary goals.[16][17]
Other
Work is underway to develop a sCO
2 closed-cycle gas turbine to operate at temperatures near 550 °C. This would have implications for bulk thermal and nuclear generation of electricity, because the supercritical properties of carbon dioxide at above 500 °C and 20 MPa enable thermal efficiencies approaching 45 percent. This could increase the electrical power produced per unit of fuel required by 40 percent or more. Given the volume of carbon fuels used in producing electricity, the environmental impact of cycle efficiency increases would be significant.[18]
Supercritical CO
2 is an emerging natural refrigerant, used in new, low carbon solutions for domestic heat pumps. Supercritical CO
2 heat pumps are commercially marketed in Asia. EcoCute systems from Japan, developed by Mayekawa, develop high temperature domestic water with small inputs of electric power by moving heat into the system from the surroundings.[19]
Supercritical CO
2 has been used since the 1980s to enhance recovery in mature oil fields.
"Clean coal" technologies are emerging that could combine such enhanced recovery methods with carbon sequestration. Using gasifiers instead of conventional furnaces, coal and water is reduced to hydrogen gas, carbon dioxide and ash. This hydrogen gas can be used to produce electrical power In combined cycle gas turbines, CO
2 is captured, compressed to the supercritical state and injected into geological storage, possibly into existing oil fields to improve yields. The unique properties of sCO
2 ensure that it remains out of the atmosphere.[20][21][22]
Supercritical CO
2 could be used as a working fluid in enhanced geothermal systems. Possible advantages compared to water include higher energy yield resulting from its lower viscosity, better chemical interaction, CO
2 storage through fluid loss and higher temperature limit. As of 2011, the concept had not been tested in the field.[23][24][25][26][27]
Aerogel production
Supercritical carbon dioxide is used in the production of silica, carbon and metal based aerogels. For example, silicon dioxide gel is formed and then exposed to sCO
2. When the CO
2 goes supercritical, all surface tension is removed, allowing the liquid to leave the aerogel and produce nanometer sized pores.[28]
Sterilization of biomedical materials
Supercritical CO
2 is an alternative for terminal sterilization of biological materials and medical devices with combination of the additive peracetic acid (PAA). Supercritical CO
2 does not sterilize the media, because it does not kill the spores of microorganisms. Moreover, this process is gentle, as the morphology, ultrastructure and protein profiles of inactivated microbes are preserved.[29]
Cleaning
Supercritical CO
2 is used in certain industrial cleaning processes.
Venus
Early Venus, with a boiling atmospheric pressure 100 times Earth's may have had a carbon dioxide ocean, in supercritical liquid state.[30] The current atmosphere, which has an average temperature of 740 K and average pressure of 92 atm (varying by elevation), is in the supercritical range, as the critical temperature of CO
2 is 304 K and the critical pressure is 72.9 atm.
See also
References
- ^ Discovery - Can Chemistry Save The World? - BBC World Service
- ^ Department of Pharmaceutical Analysis, Shenyang Pharmaceutical University, Shenyang 110016, China
- ^ Stewart, Gina (2003), "Dry Cleaning with Liquid Carbon Dioxide", Green chemistry using liquid and sCO
2, United States: Oxford University Press: 215–227{{citation}}: Unknown parameter|editors=ignored (|editor=suggested) (help) - ^ Mendiola, J.A.; Herrero, M.; Cifuentes, A.; Ibañez, E. (2007). "Use of compressed fluids for sample preparation: Food applications". Journal of Chromatography A. 1152 (1–2): 234–246. doi:10.1016/j.chroma.2007.02.046.
- ^ U.S.EPA Method 3560 Supercritical Fluid Extraction of Total Recoverable Hydrocarbons [dead link]
- ^ U.S.EPA Method 3561 Supercritical Fluid Extraction of Polycyclic Aromatic Hydrocarbons.
- ^ Use of Ozone Depleting Substances in Laboratories. TemaNord 2003:516.
- ^ Yeo, S.; Kiran, E. (2005). "Formation of polymer particles with supercritical fluids: A review". J. Supercrit. Fluids. 34: 287–308. doi:10.1016/j.supflu.2004.10.006.
- ^ Budisa, Nediljko; Schulze-Makuch, Dirk (8 August 2014). "Supercritical Carbon Dioxide and Its Potential as a Life-Sustaining Solvent in a Planetary Environment". Life. 4 (3): 331–340. doi:10.3390/life4030331.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Rubin, James B.; Taylor, Craig M. V.; Hartmann, Thomas; Paviet-Hartmann, Patricia (2003), "Enhancing the Properties of Portland Cements Using Supercritical Carbon Dioxide", Green chemistry using liquid and supercritical carbon dioxide, USA: Oxford University Press: 241–255
{{citation}}: Unknown parameter|editors=ignored (|editor=suggested) (help) - ^ Sakakura, Toshiyasu; Choi, Jun-Chul; Yasuda, Hiroyuki (13 June 2007). "Transformation of Carbon dioxide". Chemical Reviews. 107 (6). American Chemical Society: 2365–2387. doi:10.1021/cr068357u. PMID 17564481.
- ^ "Supercritical CO2 Power Cycle Developments and Commercialization: Why sCO2 can Displace Steam" (PDF).
- ^ Talbot, David (April 11, 2016). "Desk-Size Turbine Could Power a Town". MIT Technology Review. Retrieved 2016-04-13.
- ^ "Supercritical Carbon Dioxide Power Cycles Starting to Hit the Market". Breaking Energy.
- ^ "Corrosion and Erosion Behavior in sCO
2 Power Cycles" (PDF). Sandia National Laboratories. - ^ "THE EFFECT OF TEMPERATURE ON THE sCO2 COMPATIBILITY OF CONVENTIONAL STRUCTURAL ALLOYS" (PDF). The 4th International Symposium - Supercritical CO2 Power Cycles.
- ^ J. Parks, Curtis. "Corrosion of Candidate High Temperature Alloys in Supercritical Carbon Dioxide" (PDF). Ottawa-Carleton Institue for Mechanical and Aerospace Engineering.
- ^ V. Dostal, M.J. Driscoll, P. Hejzlar, A Supercritical Carbon Dioxide Cycle for Next Generation Nuclear Reactors at the Wayback Machine (archived December 27, 2010) MIT-ANP-Series, MIT-ANP-TR-100 (2004)
- ^ "Heat Pumps". Mayekawa Manufacturing Company (Mycom). Retrieved 7 February 2015.
- ^ "The Hydrogen Economy: Opportunities, Costs, Barriers, and R&D Needs", p. 84 (2004)
- ^ "FutureGen 2.0 Project". FutureGen Alliance. Archived from the original on 10 February 2015. Retrieved 7 February 2015.
{{cite web}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ Øyvind Vessia: "Fischer- Tropsch reactor fed by syngas"
- ^ K Pruess(2006), "A hot dry rock geothermal energy concept utilizing sCO
2 instead of water" - ^ Donald W. Brown(2000), "On the feasibility of using sCO
2 as heat transmission fluid in an engineered hot dry rock geothermal system" - ^ K Pruess(2007)Enhanced Geothermal Systems (EGS) comparing water with CO
2 as heat transmission fluids" - ^ J Apps(2011), "Modeling geochemical processes in enhanced geothermal systems with CO
2 as heat transfert fluid" - ^ http://earthsciences.typepad.com/blog/2011/06/achieving-carbon-sequestration-and-geothermal-energy-production-a-win-win.html ESD News and Events "Achieving Carbon Sequestration and Geothermal Energy Production: A Win-Win!"
- ^ "Aerogel.org » Supercritical Drying".
- ^ A.White, D. Burns, T.W. Christensen, "Effective terminal sterilization using supercritical carbon dioxide", J. of Biotechnology
- ^ Bolmatov, Dima (29 July 2014). "Structural Evolution of Supercritical CO2 across the Frenkel Line". Journal of Physical Chemistry Letters. 5: 2785–2790. doi:10.1021/jz5012127.
Further reading
Mukhopadhyay M. Natural extracts using supercritical carbon dioxide. United States: CRC Press, LLC; 2000 Free preview at Google Books
