Talk:Flubber (material)
| This article is rated Start-class on Wikipedia's content assessment scale. It is of interest to the following WikiProjects: | |||||||||||
| |||||||||||
Untitled
[edit]The flubber recipe is widely disseminated on the web. The specific recipe and the formula were indeed taken from the quoted website, which however belongs to an educational institution. There is also something weird about this potential copyright violation detection procedure. Arguably, if I had not added the reference to the source web-site this issue would not have arisen. So in fact, proper attribution is being penalized.
- Sorry, even a violation that might not have been detected under other circumstances is still a violation, and authors are expected not to commit violations, not merely to hide them! Also, since people aren't presumed to benefit from having posted articles on Wikipedia, the word "penalized" doesn't apply. Abiding by Wikipedia's policies and guidelines is to the benefit of its users.
- Also, when you post a note on a talk page, please sign it by placing four tildes (~~~~) at the end. They'll be converted into a signature when you save your change.
- Besides, flubber is really a fictional material from a Disney film starring Fred MacMurray some 40 or 50 years ago.—Largo Plazo (talk) 09:24, 4 November 2008 (UTC)
- Oh: I was able to find the source page via Google anyway.—Largo Plazo (talk) 09:27, 4 November 2008 (UTC)
Ok, guys I have now adapted the wording, removed the equation, so I think it should be acceptable under the guidelines. Bela Mulder (talk) 09:32, 4 November 2008 (UTC)
Largo-Plaza, thanks for all the info. It was instructive. I have to say I am impressed by the speed of the whole process. It underscores the viability of Wikipedia as a trustworthy source of knowledge. I will post the recipe on wikihow. Bela Mulder (talk) 11:53, 4 November 2008 (UTC)
Hydrogen Bonding
[edit]I have tried this experiment, thinking that it was H-bonding that caused the reaction. However, when it came to it, I found that Borax worked, and not Boric acid. Moreover, when HCl was added, it destroyed the cross-linked bonds, turning it into a less-viscous liquid. Added to that, other chemicals that I tried that should have H-bonded (including glycerol and ethane diol) didn't work either. So, there must be something unique about the Borax that makes it work, and not the others. Theory; not hydrogen bonding.
Anyone with comments can tell me whether I'm wrong or not. — Preceding unsigned comment added by TotNoob102 (talk • contribs) 22:18, 3 May 2012 (UTC)
Having completed the tests re: PVA H-bonds, I discovered that the PVA reacts with the tetrahydroxyborate (B(OH)4-); a molecule with a high reactivity. Stereochemistry played a role, as did ion-dipole and H-bonding. If you want I can send you my findings (yes, am only 16, but empirical evidence is better than superstition) and/or edit the page accordingly. TotNoob102 (talk) 09:14, 4 August 2012 (UTC)
- Hello TotNoob102. Your previous observations make sense in light of the paper I cited in the article. Indeed, there is something unique about borate. I can add a structural diagram to the article in the next week or so that shows this better, but the borate ion crosslinks the diol groups on the poly(vinyl alcohol). The hydrogen bonds occur between the borate hydroxyls and the PVA diols; borax hydrolyzes in water to form borate. There seems to be a lot more literature on the topic, but I couldn't precisely figure out why boric acid wouldn't work to cross link as well (although other authors agree, boric acid goes not produce gelling). If you could post your results or link a pdf that would be great. Scientific29 (talk) 03:21, 5 August 2012 (UTCU)
Umm, I'm not sure how to get a pdf, I did it on word and haven't tried before -.- . But i can try to summarize what i got. Bare with me. When I mixed PVA with a borax solution I got my slime, no problems. This, i thought, was due to H-bongind between PVA and boric acid. BUT, when I reacted PVA with ethan-1,2-diol (HOCH2CH2OH) and propan-1,2,3-triol (HOCH2(HOCH)CH2OH) I got no noticeable change in physical properties. The viscosity increased marginally, which i assume was due to H-bonds, but it was of little significance compared to the BRX/PVA mix. Anyway, I then looked up reactions of Borax with water and found tetrahydroxyborate to be a product. To prove (or disprove) its existence, I added HCL and NaOH to each batch of solution (PVA+BRX, PVA+EDL, PVA+PTL, all in 4 different concentrations). The BRX solution was protonated, reducing the viscosity, but did nothing to the other 2. With the NaOH, the BRX solution became really viscous (rubber-like consistency), with no reaction amongst the other 2. So, I investigated the 3-D structures using a computer program and found that the tetrahydroxyborate held on in such a way that there was an ion-dipole and 4 H-bonds occuring with 2 of the THB's hydroxyls. To be honest, the stereochemistry was close to perfect: it just fit so well. So, yeah, that's about it. I'll try to link you a pdf for clarification (once i find out how), but in the mean time, I hope that helped. Any questions, just ask. TotNoob102 (talk) 08:52, 5 August 2012 (UTC) Structure added. Scientific29 (talk) 16:19, 18 August 2012 (UTC)
I could be wrong but my guess is that this article is flawed
[edit]Its a borate ester, not some flimsy H-bonded thing. So I removed the image (which is widely propagated on the WWW, unfortunately). Hopefully I am incorrect.
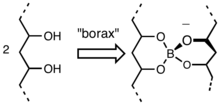
--Smokefoot (talk) 03:19, 18 February 2013 (UTC)
- The image in question comes from a 1986 chem education paper (it's from an ACS journal so it's pretty trivial to get full text) and they do discuss the possibility of a covalently bonded structure, but seem to dismiss it on the grounds that "[it] cannot be the case, however, if we consider how readily the bonds must form, break, and re- form to account for the physical properties of these borate gels. The properties of the poly(viny1 alcohol) borate gel can he rationalized by assuming that the borate cross-linkages are not fixed hut break and reform easily". I'm not exactly an expert on rheology, but their logic does seem a little hand wave-y and basically seems to come down to [paraphrasing] "it's more fluid-like than it should be if it were covalently crosslinked, therefore hydrogen-bonds" [/paraphrasing]. I'd hate to use a 26 year old chemical education publication as a definitive source on the mechanism of gelation of PVA. So in short; completely agree with the removal. (+)H3N-Protein\Chemist-CO2(-) 20:06, 18 February 2013 (UTC)
- Not to mention, those were pretty ridiculous looking hydrogen bonds. (+)H3N-Protein\Chemist-CO2(-) 20:10, 18 February 2013 (UTC)
- BTW, your image looks good and is much more consistent with current literature on PVA gels, but you need a -1 in there somewhere. ;-) (+)H3N-Protein\Chemist-CO2(-) 20:24, 18 February 2013 (UTC)
- Hello All. Full disclosure: I created the original image! Few thoughts:
- I agree the rheological explanation for hydrogen bonding over covalent bonding is hand-wavy (heuristic in kinder terms (: ), but it still seems reasonable. It seems like you're a bit more familiar with the literature, Protein Chemist. Perhaps you could guide us towards a citation for a new chemical structure? Otherwise, I can poke around later this week.
- For future reference, what would be a more standard way to present the hydrogen bonding, or did I put them in the wrong places?
- Is the above-plane oxygen atom due to the negative charge, I assume? (My chemistry is a bit rusty as you can probably see.)
- Cheers! Scientific29 (talk) 05:34, 19 February 2013 (UTC)
- Hmm.. I think I may have actually been a little premature with the PEG reference. Looks like PEG and Borate actually make a 3-armed planar borate ester. This is a little less clear cut than I originally thought. The chem ed paper implies that it makes something tetrahedral (though they prefer the H-bonded version) but doesn't really say why. This one uses the tetrahedral borate ester, but it's part of a reaction "scheme" which means it's probably intended more as a cartoon than a mechanism. So, I guess we're back where we started.. mostly. (+)H3N-Protein\Chemist-CO2(-) 11:30, 19 February 2013 (UTC)
- Hello All. Full disclosure: I created the original image! Few thoughts:

