Wikipedia:Reference desk/Archives/Science/2022 January 26
| Science desk | ||
|---|---|---|
| < January 25 | << Dec | January | Feb >> | Current desk > |
| Welcome to the Wikipedia Science Reference Desk Archives |
|---|
| The page you are currently viewing is a transcluded archive page. While you can leave answers for any questions shown below, please ask new questions on one of the current reference desk pages. |
January 26[edit]
Maximum no. of compounds[edit]
The elements which form the most compounds are carbon, which most of the compounds of carbon have hydrogen in them, and hydrogen, which is the element which can form compounds with almost all of the elements in the periodic table. If hydrogen forms the maximum number of compounds, why is a whole branch of chemistry dedicated to the compounds of carbon? Maximum number of compounds are formed by Which element? Carbon, hydrogen, or any other Huzaifa abedeen (talk) 06:17, 26 January 2022 (UTC)
- Organic chemistry is interesting because of its practical applications in biological processes. "Life chemistry" is also something of a historical accident, as in the past people held the idea that living matter had some kind of a mystical life force that made it fundamentally different from non-living matter.
- We could invent a specific field of study of hydrogen for purely intellectual interest, and invent a name for that, but what can be said about hydrogen is already pretty well covered by general chemistry.
- The number of different molecules that can be formed is pretty much an open-ended question because there are big molecules that can not be readily counted, such as DNA and proteins and other macromolecules. 85.76.87.150 (talk) 08:52, 26 January 2022 (UTC)
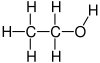
Structure of ethanol - (edit conflict) If you look at the structure of compounds, like that of ethanol or benzene, you can see that the carbon atoms can form chains and rings, and thereby the backbone of a compound. Hydrogen can only appear as fringe. --Lambiam 09:14, 26 January 2022 (UTC)
- Per Lambiam for the same reason that the study of human biology spends more of its energy on your internal organs than your hair, organic chemistry is more concerned with the carbon than the hydrogen. The carbon provides the primary structure and function of organic molecules, while the hydrogen, while there is a lot of it, is just kinda "there". You have more hairs than hearts, but I dare say your heart's function is more vital to your life than that of your hair. --Jayron32 11:51, 26 January 2022 (UTC)
- 85.76.87.150, if you think that living and non-living matter are not fundamentally different, I suggest you do some more reading. Start with life. -- Jack of Oz [pleasantries] 21:40, 26 January 2022 (UTC)
- See Wöhler synthesis, in particular the "Debates" section. See also Vitalism. Martin of Sheffield (talk) 21:50, 26 January 2022 (UTC)
- As Martin implies above, there is nothing special about the atoms, compounds, molecules, etc. that make up living things that makes them different than the exact same molecules as made from non-living sources. If you created DNA from carbon, hydrogen, oxygen, nitrogen, phosphorus, and sulfur atoms, each of which came from non-living sources, then that DNA would behave exactly like DNA created inside of your cells. The now long-debunked theory that there was some special "life force" that made living matter somehow different from non-living matter is called vitalism, and it's not a thing. The work of Friedrich Wöhler and Hermann Kolbe put an end to that thinking. --Jayron32 12:49, 27 January 2022 (UTC)
- Sure, life is different from non-life. Life has, depending on your definition, properties such as (1) birth, (2) death, (3) metabolism, (4) reproduction. The definition differs depending on who you ask.
- If you send a probe to Mars to find "life" you need to think pretty hard about what that word means, and how to differentiate between "life" and "non-life" ground samples.
- But at the molecular lever making a stand between living and not-living molecules is magical thinking. Compounds that contain carbon are not magically different those that do not.
- Thank you for your suggestion of "doing more reading". I genuinely non-sarcastically do try to do that. 85.76.87.150 (talk) 17:01, 27 January 2022 (UTC)
- The CAS database has at least 3 orders of magnitude more carbon compounds than non-carbon. Sagittarian Milky Way (talk) 21:59, 26 January 2022 (UTC)
- Carbon § Compounds: Carbon is so special because carbon atoms can bind to each other to form long chains, and these bonds between carbon atoms are strong and stable. This is why all known life is based on carbon chemistry. No other element can do this to the degree that carbon can. At Earth surface conditions, the only stable hydrogen-only compound is dihydrogen, a gas. --47.155.96.47 (talk) 00:46, 27 January 2022 (UTC)
- The strong desire to form 4 bonds, ability to bond the most common non-hermit atoms in the universe (H=1 C=4 N=3 O=2) and semi-common FClBrI (1 bond) and PS (multiple) atoms, and the ability for 1 or 2 or 3 or even "1.5" of those bonds to be used up on the same atom pair also help. Sagittarian Milky Way (talk) 01:57, 27 January 2022 (UTC)
- The table at Talk:Nonmetal gives these data for the top 7 elements found in compounds at November 2nd, 2021, according to CAS. Carbon beats hydrogen, but only just. As has already been mentioned, organic chemists focus on the chemistry of carbon, not that of hydrogen. Mike Turnbull (talk) 18:34, 1 February 2022 (UTC)
| # | Element | Count |
|---|---|---|
| 1 | C | 187,987,762 |
| 2 | H | 187,665,781 |
| 3 | N | 172,740,782 |
| 4 | O | 166,497,214 |
| 5 | S | 58,568,561 |
| 6 | F | 49,250,153 |
| 7 | Cl | 40,089,432 |
- @User:Michael D. Turnbull, but most of those have carbon, the number of compounds that don't have any carbon atoms at all is tiny in comparison. Sagittarian Milky Way (talk) 20:34, 2 February 2022 (UTC)
Tar sands tailings[edit]
Which metals are concentrated to a significant degree in the tailings from tar sands processing? In particular, do they contain high levels of metals from groups 4-6, 12 and 14? Is there a comprehensive list of metals in tar sands tailings somewhere out there? 69.181.91.208 (talk) 12:41, 26 January 2022 (UTC)
- It would, of course, depend on which tar sands are being looked at, but this has some promising leads to help with your research. --Jayron32 12:44, 26 January 2022 (UTC)
- Excellent! So, we have titanium, zirconium, vanadium, rare earths (great!), mercury (not quite what I was looking for, but still useful), arsenic (same), lead and iron (useful, but not as exciting as the first four) -- anything else? (For example, I've read somewhere that these tailings have high levels of columbium and tantalum -- any confirmation on this?) 2601:646:8A81:6070:ADA7:93DA:4B41:EBF (talk) 02:35, 29 January 2022 (UTC)

