Wohl degradation
The Wohl degradation in carbohydrate chemistry is a chain contraction method for aldoses.[1] The classic example is the conversion of glucose to arabinose as shown below. The reaction is named after the chemist Alfred Wohl.
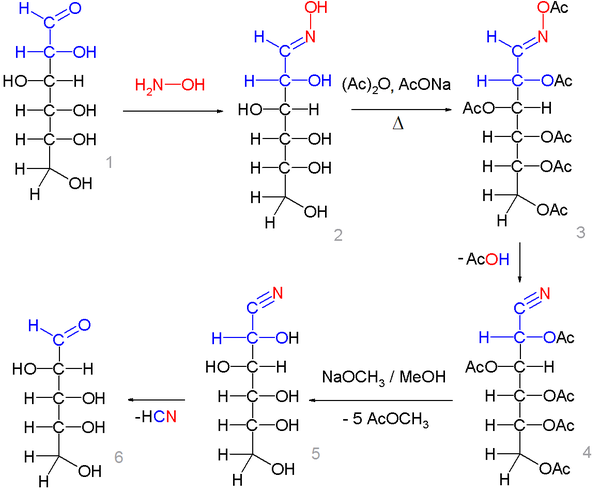
In one modification,[2][3] d-glucose is converted to the glucose oxime by reaction with hydroxylamine and sodium methoxide. In the second step the pentaacetyl glycononitrile is formed by reaction with acetic anhydride in acetic acid with sodium acetate. In this reaction step the oxime is converted into the nitrile with simultaneous conversion of all the alcohol groups to acetate groups.
In the final step sodium methoxide in methanol is added on which the nitrile group is split off as sodium cyanide with formation of a new formyl group.
Ruff-Fenton degradation
In a variation the Ruff-Fenton degradation (Otto Ruff 1898, O. Fenton 1893) converts the aldose first to the alpha-hydroxy-carboxylic acid with bromine and calcium hydroxide and then to the shortened aldose by reaction with Iron(III) sulfate and hydrogen peroxide [4]
See also
References
- ^ Wohl, A. (1893), "Abbau des Traubenzuckers", Chem. Ber., 26 (1): 730–744, doi:10.1002/cber.189302601150.
- ^ Braun, Géza (1940). "D-Arabinose". Organic Syntheses. 20: 14; Collected Volumes, vol. 3, p. 101..
- ^ Clarke, H. T.; Nagy, S. M. (1940). "Pentaacetyl d-gluconitrile". Organic Syntheses. 20: 74; Collected Volumes, vol. 3, p. 690..
- ^ Organic syntheses based on name reactions, Volume 22 Alfred Hassner,C. Stumer
