Zooplankton: Difference between revisions
Epipelagic (talk | contribs) →Microzooplankton: ref |
Epipelagic (talk | contribs) →Role in food webs: add some |
||
| Line 337: | Line 337: | ||
==Role in food webs== |
==Role in food webs== |
||
Grazing by single-celled zooplankton accounts for the majority of [[organic carbon]] loss from [[marine primary production]]. |
|||
<ref name=MendenDeuer2021>{{cite journal |doi = 10.3389/fmars.2021.695938|doi-access = free|title = Promoting Instrument Development for New Research Avenues in Ocean Science: Opening the Black Box of Grazing|year = 2021|last1 = Menden-Deuer|first1 = Susanne|last2 = Slade|first2 = Wayne Homer|last3 = Dierssen|first3 = Heidi|journal = Frontiers in Marine Science|volume = 8}} [[File:CC-BY icon.svg|50px]] Material was copied from this source, which is available under a [https://creativecommons.org/licenses/by/4.0/ Creative Commons Attribution 4.0 International License].</ref> However, zooplankton grazing remains one of the key unknowns in global predictive models of carbon flux, the [[marine food web]] structure and ecosystem characteristics, because empirical grazing measurements are sparse, resulting in poor parameterisation of grazing functions.<ref name=Stock2010>{{cite journal |doi = 10.1016/j.dsr.2009.10.006|title = Controls on the ratio of mesozooplankton production to primary production in marine ecosystems|year = 2010|last1 = Stock|first1 = Charles|last2 = Dunne|first2 = John|journal = Deep Sea Research Part I: Oceanographic Research Papers|volume = 57|issue = 1|pages = 95–112|bibcode = 2010DSRI...57...95S}}</ref><ref name=Bisson2020>{{cite journal |doi = 10.3389/fmars.2020.00505|doi-access = free|title = Diagnosing Mechanisms of Ocean Carbon Export in a Satellite-Based Food Web Model|year = 2020|last1 = Bisson|first1 = Kelsey|last2 = Siegel|first2 = David A.|last3 = Devries|first3 = Timothy|journal = Frontiers in Marine Science|volume = 7}}</ref> To overcome this critical knowledge gap, it has been suggested that a focused effort be placed on the development of instrumentation that can link changes in phytoplankton biomass or optical properties with grazing.<ref name=MendenDeuer2021 /> |
|||
Grazing is a central, rate-setting process in ocean ecosystems and a driver of [[marine biogeochemical cycling]].<ref>{{cite journal |doi = 10.1126/science.1257594|title = Rethinking the marine carbon cycle: Factoring in the multifarious lifestyles of microbes|year = 2015|last1 = Worden|first1 = A. Z.|last2 = Follows|first2 = M. J.|last3 = Giovannoni|first3 = S. J.|last4 = Wilken|first4 = S.|last5 = Zimmerman|first5 = A. E.|last6 = Keeling|first6 = P. J.|journal = Science|volume = 347|issue = 6223|pmid = 25678667|s2cid = 206560125}}</ref> In all ocean ecosystems, grazing by heterotrophic protists constitutes the single largest loss factor of marine primary production and alters particle size distributions.<ref>{{cite journal |doi = 10.1146/annurev-marine-010814-015924|title = Zooplankton and the Ocean Carbon Cycle|year = 2017|last1 = Steinberg|first1 = Deborah K.|last2 = Landry|first2 = Michael R.|journal = Annual Review of Marine Science|volume = 9|pages = 413–444|pmid = 27814033|bibcode = 2017ARMS....9..413S}}</ref> Grazing affects all pathways of export production, rendering grazing important both for surface and [[Deep carbon cycle|deep carbon]] processes.<ref>{{cite journal |doi = 10.4319/lo.2013.58.1.0173|title = Control of plankton seasonal succession by adaptive grazing|year = 2013|last1 = Mariani|first1 = Patrizio|last2 = Andersen|first2 = Ken H.|last3 = Visser|first3 = André W.|last4 = Barton|first4 = Andrew D.|last5 = Kiørboe|first5 = Thomas|journal = Limnology and Oceanography|volume = 58|issue = 1|pages = 173–184|bibcode = 2013LimOc..58..173M}}</ref> Predicting central paradigms of ocean ecosystem function, including responses to environmental change requires accurate representation of grazing in global biogeochemical, ecosystem and cross-biome-comparison models.<ref name=Stock2010 /> Several large-scale analyses have concluded that phytoplankton losses, which are dominated by grazing are the putative explanation for annual cycles in phytoplankton biomass, accumulation rates and export production.<ref>{{cite journal |doi = 10.1890/09-1207.1|title = Abandoning Sverdrup's Critical Depth Hypothesis on phytoplankton blooms|year = 2010|last1 = Behrenfeld|first1 = Michael J.|journal = Ecology|volume = 91|issue = 4|pages = 977–989|pmid = 20462113}}</ref><ref>{{cite journal |doi = 10.1038/s41467-017-02143-6|title = Floats with bio-optical sensors reveal what processes trigger the North Atlantic bloom|year = 2018|last1 = Mignot|first1 = A.|last2 = Ferrari|first2 = R.|last3 = Claustre|first3 = H.|journal = Nature Communications|volume = 9|issue = 1|page = 190|pmid = 29335403|pmc = 5768750|bibcode = 2018NatCo...9..190M}}</ref><ref name=Bisson2020 /><ref name=MendenDeuer2021 /> |
|||
<gallery mode=packed heights=330px style=float:left; caption="Pelagic food web"> |
<gallery mode=packed heights=330px style=float:left; caption="Pelagic food web"> |
||
File:Export Processes in the Ocean from Remote Sensing.jpg| [[Pelagic food web]] and the [[biological pump]]. Links among the ocean's biological pump and pelagic food web and the ability to sample these components remotely from ships, satellites, and autonomous vehicles. Light blue waters are the [[euphotic zone]], while the darker blue waters represent the [[Mesopelagic zone|twilight zone]].<ref>{{cite journal |doi=10.3389/fmars.2016.00022|title=Prediction of the Export and Fate of Global Ocean Net Primary Production: The EXPORTS Science Plan|year=2016|last1=Siegel|first1=David A.|last2=Buesseler|first2=Ken O.|last3=Behrenfeld|first3=Michael J.|last4=Benitez-Nelson|first4=Claudia R.|last5=Boss|first5=Emmanuel|last6=Brzezinski|first6=Mark A.|last7=Burd|first7=Adrian|last8=Carlson|first8=Craig A.|last9=d'Asaro|first9=Eric A.|last10=Doney|first10=Scott C.|last11=Perry|first11=Mary J.|last12=Stanley|first12=Rachel H. R.|last13=Steinberg|first13=Deborah K.|journal=Frontiers in Marine Science|volume=3|doi-access=free}} [[File:CC-BY icon.svg|50px]] Material was copied from this source, which is available under a [https://creativecommons.org/licenses/by/4.0/ Creative Commons Attribution 4.0 International License].</ref> |
File:Export Processes in the Ocean from Remote Sensing.jpg| [[Pelagic food web]] and the [[biological pump]]. Links among the ocean's biological pump and pelagic food web and the ability to sample these components remotely from ships, satellites, and autonomous vehicles. Light blue waters are the [[euphotic zone]], while the darker blue waters represent the [[Mesopelagic zone|twilight zone]].<ref>{{cite journal |doi=10.3389/fmars.2016.00022|title=Prediction of the Export and Fate of Global Ocean Net Primary Production: The EXPORTS Science Plan|year=2016|last1=Siegel|first1=David A.|last2=Buesseler|first2=Ken O.|last3=Behrenfeld|first3=Michael J.|last4=Benitez-Nelson|first4=Claudia R.|last5=Boss|first5=Emmanuel|last6=Brzezinski|first6=Mark A.|last7=Burd|first7=Adrian|last8=Carlson|first8=Craig A.|last9=d'Asaro|first9=Eric A.|last10=Doney|first10=Scott C.|last11=Perry|first11=Mary J.|last12=Stanley|first12=Rachel H. R.|last13=Steinberg|first13=Deborah K.|journal=Frontiers in Marine Science|volume=3|doi-access=free}} [[File:CC-BY icon.svg|50px]] Material was copied from this source, which is available under a [https://creativecommons.org/licenses/by/4.0/ Creative Commons Attribution 4.0 International License].</ref> |
||
</gallery> |
</gallery> |
||
[[File:Seawater constituents generated and altered by herbivorous grazing.jpg|thumb|upright=2| {{center|Seawater constituents generated and altered<br />by herbivorous zooplankton grazing{{hsp}}<ref name=MendenDeuer2021 />}}]] |
|||
{{clear}} |
{{clear}} |
||
Revision as of 06:17, 21 September 2021

| Part of a series on |
| Plankton |
|---|
 |
Zooplankton (/ˈzoʊ.əˌplæŋktən, ˈzuː(ə)-, ˈzoʊoʊ-/;[1] /ˌzoʊ.əˈplæŋktən, -tɒn/)[2] are heterotrophic (sometimes detritivorous) plankton (cf. phytoplankton). Plankton are organisms drifting in oceans, seas, and bodies of fresh water. The word zooplankton is derived from the Greek zoon (ζῴον), meaning "animal", and planktos (πλαγκτός), meaning "wanderer" or "drifter".[3] Individual zooplankton are usually microscopic, but some (such as jellyfish) are larger and visible to the naked eye.
Overview
Zooplankton are the animal component of the planktonic community ("zoo" comes from the Greek word for animal). They are heterotrophic (other-feeding), meaning they cannot produce their own food and must consume instead other plants or animals as food. In particular, this means they eat phytoplankton.
Zooplankton are generally larger than phytoplankton, mostly still microscopic but some can be seen with the naked eye. Many protozoans (single-celled protists that prey on other microscopic life) are zooplankton, including zooflagellates, foraminiferans, radiolarians, some dinoflagellates and marine microanimals. Macroscopic zooplankton include pelagic cnidarians, ctenophores, molluscs, arthropods and tunicates, as well as planktonic arrow worms and bristle worms.
Zooplankton is a categorization spanning a range of organism sizes including small protozoans and large metazoans. It includes holoplanktonic organisms whose complete life cycle lies within the plankton, as well as meroplanktonic organisms that spend part of their lives in the plankton before graduating to either the nekton or a sessile, benthic existence. Although zooplankton are primarily transported by ambient water currents, many have locomotion, used to avoid predators (as in diel vertical migration) or to increase prey encounter rate.
- Typical models featuring zooplankton
Ecologically important protozoan zooplankton groups include the foraminiferans, radiolarians and dinoflagellates (the last of these are often mixotrophic). Important metazoan zooplankton include cnidarians such as jellyfish and the Portuguese Man o' War; crustaceans such as copepods, ostracods, isopods, amphipods, mysids and krill; chaetognaths (arrow worms); molluscs such as pteropods; and chordates such as salps and juvenile fish. This wide phylogenetic range includes a similarly wide range in feeding behavior: filter feeding, predation and symbiosis with autotrophic phytoplankton as seen in corals. Zooplankton feed on bacterioplankton, phytoplankton, other zooplankton (sometimes cannibalistically), detritus (or marine snow) and even nektonic organisms. As a result, zooplankton are primarily found in surface waters where food resources (phytoplankton or other zooplankton) are abundant.
Just as any species can be limited within a geographical region, so are zooplankton. However, species of zooplankton are not dispersed uniformly or randomly within a region of the ocean. As with phytoplankton, ‘patches’ of zooplankton species exist throughout the ocean. Though few physical barriers exist above the mesopelagic, specific species of zooplankton are strictly restricted by salinity and temperature gradients; while other species can withstand wide temperature and salinity gradients.[5] Zooplankton patchiness can also be influenced by biological factors, as well as other physical factors. Biological factors include breeding, predation, concentration of phytoplankton, and vertical migration.[5] The physical factor that influences zooplankton distribution the most is mixing of the water column (upwelling and downwelling along the coast and in the open ocean) that affects nutrient availability and, in turn, phytoplankton production.[5]
Through their consumption and processing of phytoplankton and other food sources, zooplankton play a role in aquatic food webs, as a resource for consumers on higher trophic levels (including fish), and as a conduit for packaging the organic material in the biological pump. Since they are typically small, zooplankton can respond rapidly to increases in phytoplankton abundance,[clarification needed] for instance, during the spring bloom. Zooplankton are also a key link in the biomagnification of pollutants such as mercury.[6]
Zooplankton can also act as a disease reservoir. Crustacean zooplankton have been found to house the bacterium Vibrio cholerae, which causes cholera, by allowing the cholera vibrios to attach to their chitinous exoskeletons. This symbiotic relationship enhances the bacterium's ability to survive in an aquatic environment, as the exoskeleton provides the bacterium with carbon and nitrogen.[7]
Size classification
Body size has been defined as a "master trait" for plankton as it is a morphological characteristic shared by organisms across taxonomy and that characterises the functions performed by organisms in ecosystems.[8][9] It has a paramount effect on growth, reproduction, feeding strategies and mortality.[10] One of the oldest manifestations of the biogeography of traits was proposed over 170 years ago, namely Bergmann’s rule, in which field observations showed that larger species tend to be found at higher, colder latitudes.[11][12]
In the oceans, size is critical in determining trophic links in planktonic ecosystems and is thus a critical factor in regulating the efficiency of the biological carbon pump.[13] Body size is sensitive to changes in temperature due to the thermal dependence of physiological processes.[14] The plankton is mainly composed of ectotherms which are organisms that do not generate sufficient metabolic heat to elevate their body temperature, so their metabolic processes depends on external temperature.[15] Consequently, ectotherms grow more slowly and reach maturity at a larger body size in colder environments, which has long puzzled biologists because classic theories of life-history evolution predict smaller adult sizes in environments delaying growth.[16] This pattern of body size variation, known as the temperature-size rule (TSR),[17] has been observed for a wide range of ectotherms, including single-celled and multicellular species, invertebrates and vertebrates.[16][18][12]
The processes underlying the inverse relationship between body size and temperature remain to be identified.[16] Despite temperature playing a major role in shaping latitudinal variations in organism size, these patterns may also rely on complex interactions between physical, chemical and biological factors. For instance, oxygen supply plays a central role in determining the magnitude of ectothermic temperature-size responses, but it is hard to disentangle the relative effects of oxygen and temperature from field data because these two variables are often strongly inter-related in the surface ocean.[19][20][12]
Microzooplankton
Major grazers of the plankton...
Microzooplankton are the primary herbivores and nutrient regenerators in the marine food web.[21] Microzooplankton are defined as heterotrophic and mixotrophic plankton with a size range of 20–200 μm. They primarily consist of phagotrophic protists, including ciliates, dinoflagellates, and mesozooplankton nauplii.[22] As the primary consumers of marine phytoplankton, microzooplankton consume ~ 59–75% daily of the marine primary production, much larger than mesozooplankton.[23][24] Microzooplankton are also pivotal regenerators of nutrients which fuel primary production and food sources for metazoans.[24][21]
Despite their ecological importance, microzooplankton remain understudied. Routine oceanographic observations seldom monitor microzooplankton biomass or herbivory rate, although the dilution technique, an elegant method of measuring microzooplankton herbivory rate, has been developed for almost four decades (Landry and Hassett 1982). The number of observations of microzooplankton herbivory rate is around 1600 globally,[25][26] far less than that of primary productivity (> 50,000).[27] This makes validating and optimizing the grazing function of microzooplankton difficult in ocean ecosystem models.[21]
Mesozooplankton
Macrozooplankton
Taxonomic groups
Protozoa
Protozoans are protists that feed on organic matter such as other microorganisms or organic tissues and debris.[28][29] Historically, the protozoa were regarded as "one-celled animals", because they often possess animal-like behaviours, such as motility and predation, and lack a cell wall, as found in plants and many algae.[30][31] Although the traditional practice of grouping protozoa with animals is no longer considered valid, the term continues to be used in a loose way to identify single-celled organisms that can move independently and feed by heterotrophy.
Marine protozoans include zooflagellates, foraminiferans, radiolarians and some dinoflagellates.
Radiolarians
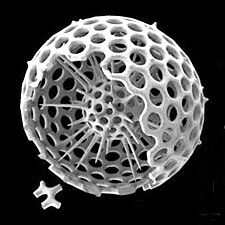
Radiolarians are unicellular predatory protists encased in elaborate globular shells usually made of silica and pierced with holes. Their name comes from the Latin for "radius". They catch prey by extending parts of their body through the holes. As with the silica frustules of diatoms, radiolarian shells can sink to the ocean floor when radiolarians die and become preserved as part of the ocean sediment. These remains, as microfossils, provide valuable information about past oceanic conditions.[32]
-
Like diatoms, radiolarians come in many shapes
-
Also like diatoms, radiolarian shells are usually made of silicate
-
However acantharian radiolarians have shells made from strontium sulfate crystals
-
Cutaway schematic diagram of a spherical radiolarian shell
| External videos | |
|---|---|
Foraminiferans
Like radiolarians, foraminiferans (forams for short) are single-celled predatory protists, also protected with shells that have holes in them. Their name comes from the Latin for "hole bearers". Their shells, often called tests, are chambered (forams add more chambers as they grow). The shells are usually made of calcite, but are sometimes made of agglutinated sediment particles or chiton, and (rarely) of silica. Most forams are benthic, but about 40 species are planktic.[33] They are widely researched with well established fossil records which allow scientists to infer a lot about past environments and climates.[32]
-
section showing chambers of a spiral foram
-
Live Ammonia tepida streaming granular ectoplasm for catching food
-
Group of planktonic forams
| External videos | |
|---|---|
Amoeba
-
Naked amoeba sketch showing food vacuoles and ingested diatom
-
Shell or test of a testate amoeba, Arcella sp.
-
Xenogenic testate amoeba covered in diatoms
Ciliates
-
Holophyra ovum
-
This ciliate is digesting cyanobacteria. The mouth is at the bottom right.
Dinoflagellates
Dinoflagellates are part of the algae group, and form a phylum of unicellular flagellates with about 2,000 marine species.[35] The name comes from the Greek "dinos" meaning whirling and the Latin "flagellum" meaning a whip or lash. This refers to the two whip-like attachments (flagella) used for forward movement. Most dinoflagellates are protected with red-brown, cellulose armour. Like other phytoplankton, dinoflagellates are r-strategists which under right conditions can bloom and create red tides. Excavates may be the most basal flagellate lineage.[36]
-
Gyrodinium, one of the few naked dinoflagellates which lack armour
-
The dinoflagellate Protoperidinium extrudes a large feeding veil to capture prey
-
Nassellarian radiolarians can be in symbiosis with dinoflagellates
Dinoflagellates often live in symbiosis with other organisms. Many nassellarian radiolarians house dinoflagellate symbionts within their tests.[37] The nassellarian provides ammonium and carbon dioxide for the dinoflagellate, while the dinoflagellate provides the nassellarian with a mucous membrane useful for hunting and protection against harmful invaders.[38] There is evidence from DNA analysis that dinoflagellate symbiosis with radiolarians evolved independently from other dinoflagellate symbioses, such as with foraminifera.[39]
-
Tripos muelleri is recognisable by its U-shaped horns
-
Karenia brevis produces red tides highly toxic to humans[41]
Mixotrophs
A mixotroph is an organism that can use a mix of different sources of energy and carbon, instead of having a single trophic mode on the continuum from complete autotrophy at one end to heterotrophy at the other. It is estimated that mixotrophs comprise more than half of all microscopic plankton.[42] There are two types of eukaryotic mixotrophs: those with their own chloroplasts, and those with endosymbionts—and others that acquire them through kleptoplasty or by enslaving the entire phototrophic cell.[43]
The distinction between plants and animals often breaks down in very small organisms. Possible combinations are photo- and chemotrophy, litho- and organotrophy, auto- and heterotrophy or other combinations of these. Mixotrophs can be either eukaryotic or prokaryotic.[44] They can take advantage of different environmental conditions.[45]
Many marine microzooplankton are mixotrophic, which means they could also be classified as phytoplankton. Recent studies of marine microzooplankton found 30–45% of the ciliate abundance was mixotrophic, and up to 65% of the amoeboid, foram and radiolarian biomass was mixotrophic.[46]
Mixotrophic zooplankton that combine phototrophy and heterotrophy – table based on Stoecker et. al., 2017 [47]
| ||||||
|---|---|---|---|---|---|---|
| Description | Example | Further examples | ||||
| Called nonconstitutive mixotrophs by Mitra et al., 2016.[48] Zooplankton that are photosynthetic: microzooplankton or metazoan zooplankton that acquire phototrophy through chloroplast retentiona or maintenance of algal endosymbionts. | ||||||
| Generalists | Protists that retain chloroplasts and rarely other organelles from many algal taxa | 
|
Most oligotrich ciliates that retain plastidsa | |||
| Specialists | 1. Protists that retain chloroplasts and sometimes other organelles from one algal species or very closely related algal species | 
|
Dinophysis acuminata | Dinophysis spp. Myrionecta rubra | ||
| 2. Protists or zooplankton with algal endosymbionts of only one algal species or very closely related algal species | 
|
Noctiluca scintillans | Metazooplankton with algal endosymbionts Most mixotrophic Rhizaria (Acantharea, Polycystinea, and Foraminifera) Green Noctiluca scintillans | |||
| aChloroplast (or plastid) retention = sequestration = enslavement. Some plastid-retaining species also retain other organelles and prey cytoplasm. | ||||||
Phaeocystis species are endosymbionts to acantharian radiolarians.[49][50] Phaeocystis is an important algal genus found as part of the marine phytoplankton around the world. It has a polymorphic life cycle, ranging from free-living cells to large colonies.[51] It has the ability to form floating colonies, where hundreds of cells are embedded in a gel matrix, which can increase massively in size during blooms.[52] As a result, Phaeocystis is an important contributor to the marine carbon[53] and sulfur cycles.[54]
- Mixoplankton
-
Tintinnid ciliate Favella
-
Euglena mutabilis, a photosynthetic flagellate
-
Zoochlorellae (green) living inside the ciliate Stichotricha secunda
-
The dinoflagellate Dinophysis acuta
A number of forams are mixotrophic. These have unicellular algae as endosymbionts, from diverse lineages such as the green algae, red algae, golden algae, diatoms, and dinoflagellates.[33] Mixotrophic foraminifers are particularly common in nutrient-poor oceanic waters.[55] Some forams are kleptoplastic, retaining chloroplasts from ingested algae to conduct photosynthesis.[56]
By trophic orientation dinoflagellates are all over the place. Some dinoflagellates are known to be photosynthetic, but a large fraction of these are in fact mixotrophic, combining photosynthesis with ingestion of prey (phagotrophy).[57] Some species are endosymbionts of marine animals and other protists, and play an important part in the biology of coral reefs. Others predate other protozoa, and a few forms are parasitic. Many dinoflagellates are mixotrophic and could also be classified as phytoplankton. The toxic dinoflagellate Dinophysis acuta acquire chloroplasts from its prey. "It cannot catch the cryptophytes by itself, and instead relies on ingesting ciliates such as the red Myrionecta rubra, which sequester their chloroplasts from a specific cryptophyte clade (Geminigera/Plagioselmis/Teleaulax)".[47]
Metazoa (animals)

Copepods are typically 1 to 2 mm long with a teardrop-shaped bodies. Like all crustaceans, their bodies are divided into three sections: head, thorax, and abdomen, with two pairs of antennae; the first pair is often long and prominent. They have a tough exoskeleton made of calcium carbonate and usually have a single red eye in the centre of their transparent head.[58] About 13,000 species of copepods are known, of which about 10,200 are marine.[59][60] They are usually among the more dominant members of the zooplankton.[61]
Holoplankton and meroplankton
Ichthyoplankton
- Ichthyoplankton
-
Salmon eggs
-
Juvenile planktonic squid
-
Ocean sunfish larvae (2.7mm)
-
Boxfish larva
Gelatinous zooplankton
Gelatinous zooplankton include ctenophores, medusae, salps, and Chaetognatha in coastal waters. Jellyfish are slow swimmers, and most species form part of the plankton. Traditionally jellyfish have been viewed as trophic dead ends, minor players in the marine food web, gelatinous organisms with a body plan largely based on water that offers little nutritional value or interest for other organisms apart from a few specialised predators such as the ocean sunfish and the leatherback sea turtle.[62][63] That view has recently been challenged. Jellyfish, and more gelatinous zooplankton in general, which include salps and ctenophores, are very diverse, fragile with no hard parts, difficult to see and monitor, subject to rapid population swings and often live inconveniently far from shore or deep in the ocean. It is difficult for scientists to detect and analyse jellyfish in the guts of predators, since they turn to mush when eaten and are rapidly digested.[62] But jellyfish bloom in vast numbers, and it has been shown they form major components in the diets of tuna, spearfish and swordfish as well as various birds and invertebrates such as octopus, sea cucumbers, crabs and amphipods.[64][63] "Despite their low energy density, the contribution of jellyfish to the energy budgets of predators may be much greater than assumed because of rapid digestion, low capture costs, availability, and selective feeding on the more energy-rich components. Feeding on jellyfish may make marine predators susceptible to ingestion of plastics."[63] According to a 2017 study, narcomedusae consume the greatest diversity of mesopelagic prey, followed by physonect siphonophores, ctenophores and cephalopods.[65] The importance of the so-called "jelly web" is only beginning to be understood, but it seems medusae, ctenophores and siphonophores can be key predators in deep pelagic food webs with ecological impacts similar to predator fish and squid. Traditionally gelatinous predators were thought ineffectual providers of marine trophic pathways, but they appear to have substantial and integral roles in deep pelagic food webs.[65]
Role in food webs
Grazing by single-celled zooplankton accounts for the majority of organic carbon loss from marine primary production. [66] However, zooplankton grazing remains one of the key unknowns in global predictive models of carbon flux, the marine food web structure and ecosystem characteristics, because empirical grazing measurements are sparse, resulting in poor parameterisation of grazing functions.[67][68] To overcome this critical knowledge gap, it has been suggested that a focused effort be placed on the development of instrumentation that can link changes in phytoplankton biomass or optical properties with grazing.[66]
Grazing is a central, rate-setting process in ocean ecosystems and a driver of marine biogeochemical cycling.[69] In all ocean ecosystems, grazing by heterotrophic protists constitutes the single largest loss factor of marine primary production and alters particle size distributions.[70] Grazing affects all pathways of export production, rendering grazing important both for surface and deep carbon processes.[71] Predicting central paradigms of ocean ecosystem function, including responses to environmental change requires accurate representation of grazing in global biogeochemical, ecosystem and cross-biome-comparison models.[67] Several large-scale analyses have concluded that phytoplankton losses, which are dominated by grazing are the putative explanation for annual cycles in phytoplankton biomass, accumulation rates and export production.[72][73][68][66]
- Pelagic food web
-
Pelagic food web and the biological pump. Links among the ocean's biological pump and pelagic food web and the ability to sample these components remotely from ships, satellites, and autonomous vehicles. Light blue waters are the euphotic zone, while the darker blue waters represent the twilight zone.[74]

by herbivorous zooplankton grazing [66]
Role in biogeochemistry
In addition to linking primary producers to higher trophic levels in marine food webs, zooplankton also play an important role as “recyclers” of carbon and other nutrients that significantly impact marine biogeochemical cycles, including the biological pump. This is particularly important in the oligotrophic waters of the open ocean. Through sloppy feeding, excretion, egestion, and leaching of fecal pellets, zooplankton release dissolved organic matter (DOM) which controls DOM cycling and supports the microbial loop. Absorption efficiency, respiration, and prey size all further complicate how zooplankton are able to transform and deliver carbon to the deep ocean.[75]
Sloppy feeding and release of DOM
Excretion and sloppy feeding (the physical breakdown of food source) make up 80% and 20% of crustacean zooplankton-mediated DOM release respectively.[76] In the same study, fecal pellet leaching was found to be an insignificant contributor. For protozoan grazers, DOM is released primarily through excretion and egestion and gelatinous zooplankton can also release DOM through the production of mucus. Leaching of fecal pellets can extend from hours to days after initial egestion and its effects can vary depending on food concentration and quality.[77][78] Various factors can affect how much DOM is released from zooplankton individuals or populations. Absorption efficiency (AE) is the proportion of food absorbed by plankton that determines how available the consumed organic materials are in meeting the required physiological demands.[75] Depending on the feeding rate and prey composition, variations in AE may lead to variations in fecal pellet production, and thus regulates how much organic material is recycled back to the marine environment. Low feeding rates typically lead to high AE and small, dense pellets, while high feeding rates typically lead to low AE and larger pellets with more organic content. Another contributing factor to DOM release is respiration rate. Physical factors such as oxygen availability, pH, and light conditions may affect overall oxygen consumption and how much carbon is loss from zooplankton in the form of respired CO2. The relative sizes of zooplankton and prey also mediate how much carbon is released via sloppy feeding. Smaller prey are ingested whole, whereas larger prey may be fed on more “sloppily”, that is more biomatter is released through inefficient consumption.[79][80] There is also evidence that diet composition can impact nutrient release, with carnivorous diets releasing more dissolved organic carbon (DOC) and ammonium than omnivorous diets.[77]
Naturally iron-fertilized
High nutrient, low chlorophyll

DOC = dissolved organic carbon
POC = particulate organic carbon
Adapted from Møller et al. (2005),[82]
Saba et al. (2009)[83] and Steinberg et al. (2017).[75]
Carbon export
Zooplankton play a critical role in supporting the ocean's biological pump through various forms of carbon export, including the production of fecal pellets, mucous feeding webs, molts, and carcasses. Fecal pellets are estimated to be a large contributor to this export, with copepod size rather than abundance expected to determine how much carbon actually reaches the ocean floor. The importance of fecal pellets can vary both by time and location. For example, zooplankton bloom events can produce larger quantities of fecal pellets, resulting in greater measures of carbon export. Additionally, as fecal pellets sink, they are microbial reworked by microbes in the water column, which can thus alter the carbon composition of the pellet. This affects how much carbon is recycled in the euphotic zone and how much reaches depth. Fecal pellet contribution to carbon export is likely underestimated; however, new advances in quantifying this production are currently being developed, including the use of isotopic signatures of amino acids to characterize how much carbon is being exported via zooplankton fecal pellet production.[84] Carcasses are also gaining recognition as being important contributors to carbon export. Jelly falls – the mass sinking of gelatinous zooplankton carcasses – occur across the world as a result of large blooms. Because of their large size, these gelatinous zooplankton are expected to hold a larger carbon content, making their sinking carcasses a potentially important source of food for benthic organisms.[75]
See also
- Bacterioplankton
- Biological pump
- Census of Marine Zooplankton
- Diel vertical migration
- Gelatinous zooplankton
- Ocean acidification
- Phytoplankton
- Plankton
- Primary production
- Thin layers (oceanography)
References
- ^ "zooplankton". Oxford Dictionaries UK English Dictionary. Oxford University Press. n.d. Retrieved 2016-01-20.
- ^ "zooplankton". Merriam-Webster.com Dictionary. Merriam-Webster.
- ^ Thurman, H. V. (1997). Introductory Oceanography. New Jersey, USA: Prentice Hall College. ISBN 978-0-13-262072-7.
- ^ Everett, J.D., Baird, M.E., Buchanan, P., Bulman, C., Davies, C., Downie, R., Griffiths, C., Heneghan, R., Kloser, R.J., Laiolo, L. and Lara-Lopez, A. (2017) "Modeling what we sample and sampling what we model: challenges for zooplankton model assessment". Frontiers in Marine Science, 4: 77. doi:10.3389/fmars.2017.00077.
 Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License.
Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License.
- ^ a b c Lalli, C.M. & Parsons, T.R. (1993). Biological Oceanography An Introduction. Burlington, MA: Elsevier. p. 314. ISBN 978-0-7506-3384-0.
- ^ "How We Do Things at IISD-ELA: Researching Mercury". IISD. 2017-04-05. Retrieved 2020-07-06.
- ^ Jude, B.A.; Kirn, T.J.; Taylor R.K. (2005). "A colonization factor links Vibrio cholerae environmental survival and human infection". Nature. 438 (7069): 863–6. Bibcode:2005Natur.438..863K. doi:10.1038/nature04249. PMID 16341015. S2CID 1964530.
- ^ Litchman, Elena; Ohman, Mark D.; Kiørboe, Thomas (2013). "Trait-based approaches to zooplankton communities". Journal of Plankton Research. 35 (3): 473–484. doi:10.1093/plankt/fbt019.
- ^ Kiørboe, Thomas; Hirst, Andrew G. (2014). "Shifts in Mass Scaling of Respiration, Feeding, and Growth Rates across Life-Form Transitions in Marine Pelagic Organisms". The American Naturalist. 183 (4): E118–E130. doi:10.1086/675241. PMID 24642502. S2CID 15891709.
- ^ Andersen, K.H.; Berge, T.; Gonçalves, R.J.; Hartvig, M.; Heuschele, J.; Hylander, S.; Jacobsen, N.S.; Lindemann, C.; Martens, E.A.; Neuheimer, A.B.; Olsson, K.; Palacz, A.; Prowe, A.E.F.; Sainmont, J.; Traving, S.J.; Visser, A.W.; Wadhwa, N.; Kiørboe, T. (2016). "Characteristic Sizes of Life in the Oceans, from Bacteria to Whales" (PDF). Annual Review of Marine Science. 8: 217–241. Bibcode:2016ARMS....8..217A. doi:10.1146/annurev-marine-122414-034144. PMID 26163011.
- ^ Bergmann, Carl (1847). "Über die Verhältnisse der Wärmeökonomie der Thiere zu ihrer Grösse". Göttinger Studien. 3 (1): 595–708.
- ^ a b c Brandão, Manoela C.; et al. (2021). "Macroscale patterns of oceanic zooplankton composition and size structure". Scientific Reports. 11 (1): 15714. doi:10.1038/s41598-021-94615-5. PMC 8333327. PMID 34344925.
 Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License.
Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License.
- ^ Woodson, C. Brock; Schramski, John R.; Joye, Samantha B. (2018). "A unifying theory for top-heavy ecosystem structure in the ocean". Nature Communications. 9 (1): 23. Bibcode:2018NatCo...9...23W. doi:10.1038/s41467-017-02450-y. PMC 5750233. PMID 29295998.
- ^ Brown, James H.; Gillooly, James F.; Allen, Andrew P.; Savage, Van M.; West, Geoffrey B. (2004). "Toward a Metabolic Theory of Ecology". Ecology. 85 (7): 1771–1789. doi:10.1890/03-9000.
- ^ Gardner, Janet L.; Peters, Anne; Kearney, Michael R.; Joseph, Leo; Heinsohn, Robert (2011). "Declining body size: A third universal response to warming?". Trends in Ecology & Evolution. 26 (6): 285–291. doi:10.1016/j.tree.2011.03.005. PMID 21470708.
- ^ a b c Angilletta, M. J.; Steury, T. D.; Sears, M. W. (2004). "Temperature, Growth Rate, and Body Size in Ectotherms: Fitting Pieces of a Life-History Puzzle". Integrative and Comparative Biology. 44 (6): 498–509. doi:10.1093/icb/44.6.498. PMID 21676736.
- ^ Atkinson, D. (1994). Temperature and Organism Size—A Biological Law for Ectotherms?. Advances in Ecological Research. Vol. 25. pp. 1–58. doi:10.1016/S0065-2504(08)60212-3. ISBN 9780120139255.
- ^ Atkinson, David; Sibly, Richard M. (1997). "Why are organisms usually bigger in colder environments? Making sense of a life history puzzle". Trends in Ecology & Evolution. 12 (6): 235–239. doi:10.1016/S0169-5347(97)01058-6. PMID 21238056.
- ^ Sunagawa, S.; et al. (2015). "Structure and function of the global ocean microbiome". Science. 348 (6237). doi:10.1126/science.1261359. hdl:10261/117712. PMID 25999513. S2CID 206562917.
- ^ Audzijonyte, Asta; Barneche, Diego R.; Baudron, Alan R.; Belmaker, Jonathan; Clark, Timothy D.; Marshall, C. Tara; Morrongiello, John R.; Van Rijn, Itai (2019). "Is oxygen limitation in warming waters a valid mechanism to explain decreased body sizes in aquatic ectotherms?". Global Ecology and Biogeography. 28 (2): 64–77. doi:10.1111/geb.12847.
- ^ a b c Liu, Kailin; Chen, Bingzhang; Zheng, Liping; Su, Suhong; Huang, Bangqin; Chen, Mianrun; Liu, Hongbin (2021). "What controls microzooplankton biomass and herbivory rate across marginal seas of China?". Limnology and Oceanography. 66 (1): 61–75. Bibcode:2021LimOc..66...61L. doi:10.1002/lno.11588. S2CID 224916151.
 Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License.
Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License.
- ^ Sieburth, John McN.; Smetacek, Victor; Lenz, Jürgen (1978). "Pelagic ecosystem structure: Heterotrophic compartments of the plankton and their relationship to plankton size fractions 1". Limnology and Oceanography. 23 (6): 1256–1263. Bibcode:1978LimOc..23.1256S. doi:10.4319/lo.1978.23.6.1256.
- ^ Calbet, Albert; Landry, Michael R. (2004). "Phytoplankton growth, microzooplankton grazing, and carbon cycling in marine systems". Limnology and Oceanography. 49 (1): 51–57. Bibcode:2004LimOc..49...51C. doi:10.4319/lo.2004.49.1.0051.
- ^ a b Calbet, Albert (2008). "The trophic roles of microzooplankton in marine systems". ICES Journal of Marine Science. 65 (3): 325–331. doi:10.1093/icesjms/fsn013.
- ^ Chen, Bingzhang; Landry, Michael R.; Huang, Bangqin; Liu, Hongbin (2012). "Does warming enhance the effect of microzooplankton grazing on marine phytoplankton in the ocean?". Limnology and Oceanography. 57 (2): 519–526. Bibcode:2012LimOc..57..519C. doi:10.4319/lo.2012.57.2.0519.
- ^ Schmoker, Claire; Hernández-León, Santiago; Calbet, Albert (2013). "Microzooplankton grazing in the oceans: Impacts, data variability, knowledge gaps and future directions". Journal of Plankton Research. 35 (4): 691–706. doi:10.1093/plankt/fbt023.
- ^ Buitenhuis, Erik T.; Hashioka, Taketo; Quéré, Corinne Le (2013). "Combined constraints on global ocean primary production using observations and models". Global Biogeochemical Cycles. 27 (3): 847–858. Bibcode:2013GBioC..27..847B. doi:10.1002/gbc.20074.
- ^ Panno, Joseph (14 May 2014). The Cell: Evolution of the First Organism. Infobase Publishing. ISBN 9780816067367.
- ^ Bertrand, Jean-Claude; Caumette, Pierre; Lebaron, Philippe; Matheron, Robert; Normand, Philippe; Sime-Ngando, Télesphore (2015-01-26). Environmental Microbiology: Fundamentals and Applications: Microbial Ecology. Springer. ISBN 9789401791182.
- ^ Madigan, Michael T. (2012). Brock Biology of Microorganisms. Benjamin Cummings. ISBN 9780321649638.
- ^ Yaeger, Robert G. (1996). Protozoa: Structure, Classification, Growth, and Development. NCBI. ISBN 9780963117212. PMID 21413323. Retrieved 2018-03-23.
- ^ a b Wassilieff, Maggy (2006) "Plankton - Animal plankton", Te Ara - the Encyclopedia of New Zealand. Accessed: 2 November 2019.
- ^ a b Hemleben, C.; Anderson, O.R.; Spindler, M. (1989). Modern Planktonic Foraminifera. Springer-Verlag. ISBN 978-3-540-96815-3.
- ^ Foraminifera: History of Study, University College London. Retrieved: 18 November 2019.
- ^ Gómez F (2012). "A checklist and classification of living dinoflagellates (Dinoflagellata, Alveolata)". CICIMAR Océanides. 27 (1): 65–140. doi:10.37543/oceanides.v27i1.111.
- ^ Dawson, Scott C; Paredez, Alexander R (2013). "Alternative cytoskeletal landscapes: cytoskeletal novelty and evolution in basal excavate protists". Current Opinion in Cell Biology. 25 (1): 134–141. doi:10.1016/j.ceb.2012.11.005. PMC 4927265. PMID 23312067.
- ^ Boltovskoy, Demetrio; Anderson, O. Roger; Correa, Nancy M. (2017). Handbook of the Protists. Springer, Cham. pp. 731–763. doi:10.1007/978-3-319-28149-0_19. ISBN 9783319281476.
- ^ Anderson, O. R. (1983). Radiolaria. Springer Science & Business Media.
- ^ Gast, R. J.; Caron, D. A. (1996-11-01). "Molecular phylogeny of symbiotic dinoflagellates from planktonic foraminifera and radiolaria". Molecular Biology and Evolution. 13 (9): 1192–1197. doi:10.1093/oxfordjournals.molbev.a025684. ISSN 0737-4038. PMID 8896371.
- ^ "Protozoa Infecting Gills and Skin". The Merck Veterinary Manual. Archived from the original on 3 March 2016. Retrieved 4 November 2019.
- ^ Brand, Larry E.; Campbell, Lisa; Bresnan, Eileen (2012). "Karenia: The biology and ecology of a toxic genus". Harmful Algae. 14: 156–178. doi:10.1016/j.hal.2011.10.020.
- ^ Beware the mixotrophs - they can destroy entire ecosystems 'in a matter of hours'
- ^ Microscopic body snatchers infest our oceans - Phys.org
- ^ Eiler A (December 2006). "Evidence for the Ubiquity of Mixotrophic Bacteria in the Upper Ocean: Implications and Consequences". Appl Environ Microbiol. 72 (12): 7431–7. Bibcode:2006ApEnM..72.7431E. doi:10.1128/AEM.01559-06. PMC 1694265. PMID 17028233.
- ^ Katechakis A, Stibor H (July 2006). "The mixotroph Ochromonas tuberculata may invade and suppress specialist phago- and phototroph plankton communities depending on nutrient conditions". Oecologia. 148 (4): 692–701. Bibcode:2006Oecol.148..692K. doi:10.1007/s00442-006-0413-4. PMID 16568278. S2CID 22837754.
- ^ Leles, S.G.; Mitra, A.; Flynn, K.J.; Stoecker, D.K.; Hansen, P.J.; Calbet, A.; McManus, G.B.; Sanders, R.W.; Caron, D.A.; Not, F.; Hallegraeff, G.M. (2017). "Oceanic protists with different forms of acquired phototrophy display contrasting biogeographies and abundance". Proceedings of the Royal Society B: Biological Sciences. 284 (1860): 20170664. doi:10.1098/rspb.2017.0664. PMC 5563798. PMID 28768886.
- ^ a b Stoecker, D.K.; Hansen, P.J.; Caron, D.A.; Mitra, A. (2017). "Mixotrophy in the marine plankton" (PDF). Annual Review of Marine Science. 9: 311–335. Bibcode:2017ARMS....9..311S. doi:10.1146/annurev-marine-010816-060617. PMID 27483121. S2CID 25579538. Archived from the original (PDF) on 2019-02-27.
- ^ Mitra, A; Flynn, KJ; Tillmann, U; Raven, J; Caron, D; et al. (2016). "Defining planktonic protist functional groups on mechanisms for energy and nutrient acquisition; incorporation of diverse mixotrophic strategies". Protist. 167 (2): 106–20. doi:10.1016/j.protis.2016.01.003. PMID 26927496.
- ^ Decelle, Johan; Simó, Rafel; Galí, Martí; Vargas, Colomban de; Colin, Sébastien; Desdevises, Yves; Bittner, Lucie; Probert, Ian; Not, Fabrice (2012-10-30). "An original mode of symbiosis in open ocean plankton". Proceedings of the National Academy of Sciences. 109 (44): 18000–18005. Bibcode:2012PNAS..10918000D. doi:10.1073/pnas.1212303109. ISSN 0027-8424. PMC 3497740. PMID 23071304.
- ^ Mars Brisbin, Margaret; Grossmann, Mary M.; Mesrop, Lisa Y.; Mitarai, Satoshi (2018). "Intra-host Symbiont Diversity and Extended Symbiont Maintenance in Photosymbiotic Acantharea (Clade F)". Frontiers in Microbiology. 9: 1998. doi:10.3389/fmicb.2018.01998. ISSN 1664-302X. PMC 6120437. PMID 30210473.
- ^ Schoemann, Véronique; Becquevort, Sylvie; Stefels, Jacqueline; Rousseau, Véronique; Lancelot, Christiane (2005-01-01). "Phaeocystis blooms in the global ocean and their controlling mechanisms: a review". Journal of Sea Research. Iron Resources and Oceanic Nutrients - Advancement of Global Environmental Simulations. 53 (1–2): 43–66. Bibcode:2005JSR....53...43S. CiteSeerX 10.1.1.319.9563. doi:10.1016/j.seares.2004.01.008.
- ^ "Welcome to the Phaeocystis antarctica genome sequencing project homepage".
- ^ DiTullio, G. R.; Grebmeier, J. M.; Arrigo, K. R.; Lizotte, M. P.; Robinson, D. H.; Leventer, A.; Barry, J. P.; VanWoert, M. L.; Dunbar, R. B. (2000). "Rapid and early export of Phaeocystis antarctica blooms in the Ross Sea, Antarctica". Nature. 404 (6778): 595–598. Bibcode:2000Natur.404..595D. doi:10.1038/35007061. PMID 10766240. S2CID 4409009.
- ^ J, Stefels; L, Dijkhuizen; WWC, Gieskes (1995-07-20). "DMSP-lyase activity in a spring phytoplankton bloom off the Dutch coast, related to Phaeocystis sp. abundance" (PDF). Marine Ecology Progress Series. 123: 235–243. Bibcode:1995MEPS..123..235S. doi:10.3354/meps123235.
- ^ Advances in Microbial Ecology, Volum 11
- ^ Bernhard, J. M.; Bowser, S.M. (1999). "Benthic Foraminifera of dysoxic sediments: chloroplast sequestration and functional morphology". Earth-Science Reviews. 46 (1): 149–165. Bibcode:1999ESRv...46..149B. doi:10.1016/S0012-8252(99)00017-3.
- ^ Stoecker DK (1999). "Mixotrophy among Dinoflagellates". The Journal of Eukaryotic Microbiology. 46 (4): 397–401. doi:10.1111/j.1550-7408.1999.tb04619.x. S2CID 83885629.
- ^ Robert D. Barnes (1982). Invertebrate Zoology. Philadelphia, Pennsylvania: Holt-Saunders International. pp. 683–692. ISBN 978-0-03-056747-6.
- ^ "WoRMS - World Register of Marine Species - Copepoda". www.marinespecies.org. Archived from the original on 2019-06-30. Retrieved 2019-06-28.
- ^ Geoff A. Boxhall; Danielle Defaye (2008). "Global diversity of copepods (Crustacea: Copepoda) in freshwater". Hydrobiologia. 595 (1): 195–207. doi:10.1007/s10750-007-9014-4. S2CID 31727589.
- ^ Johannes Dürbaum; Thorsten Künnemann (November 5, 1997). "Biology of Copepods: An Introduction". Carl von Ossietzky University of Oldenburg. Archived from the original on May 26, 2010. Retrieved December 8, 2009.
- ^ a b Hamilton, G. (2016) "The secret lives of jellyfish: long regarded as minor players in ocean ecology, jellyfish are actually important parts of the marine food web". Nature, 531(7595): 432-435. doi:10.1038/531432a
- ^ a b c Hays, G.C., Doyle, T.K. and Houghton, J.D. (2018) "A paradigm shift in the trophic importance of jellyfish?" Trends in ecology & evolution, 33(11): 874-884. doi:10.1016/j.tree.2018.09.001
- ^ Cardona, L., De Quevedo, I.Á., Borrell, A. and Aguilar, A. (2012) "Massive consumption of gelatinous plankton by Mediterranean apex predators". PLOS ONE, 7(3): e31329. doi:10.1371/journal.pone.0031329
- ^ a b Choy, C.A., Haddock, S.H. and Robison, B.H. (2017) "Deep pelagic food web structure as revealed by in situ feeding observations". Proceedings of the Royal Society B: Biological Sciences, 284(1868): 20172116. doi:10.1098/rspb.2017.2116.
 Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License.
Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License.
- ^ a b c d Menden-Deuer, Susanne; Slade, Wayne Homer; Dierssen, Heidi (2021). "Promoting Instrument Development for New Research Avenues in Ocean Science: Opening the Black Box of Grazing". Frontiers in Marine Science. 8. doi:10.3389/fmars.2021.695938.
 Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License.
Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License.
- ^ a b Stock, Charles; Dunne, John (2010). "Controls on the ratio of mesozooplankton production to primary production in marine ecosystems". Deep Sea Research Part I: Oceanographic Research Papers. 57 (1): 95–112. Bibcode:2010DSRI...57...95S. doi:10.1016/j.dsr.2009.10.006.
- ^ a b Bisson, Kelsey; Siegel, David A.; Devries, Timothy (2020). "Diagnosing Mechanisms of Ocean Carbon Export in a Satellite-Based Food Web Model". Frontiers in Marine Science. 7. doi:10.3389/fmars.2020.00505.
- ^ Worden, A. Z.; Follows, M. J.; Giovannoni, S. J.; Wilken, S.; Zimmerman, A. E.; Keeling, P. J. (2015). "Rethinking the marine carbon cycle: Factoring in the multifarious lifestyles of microbes". Science. 347 (6223). doi:10.1126/science.1257594. PMID 25678667. S2CID 206560125.
- ^ Steinberg, Deborah K.; Landry, Michael R. (2017). "Zooplankton and the Ocean Carbon Cycle". Annual Review of Marine Science. 9: 413–444. Bibcode:2017ARMS....9..413S. doi:10.1146/annurev-marine-010814-015924. PMID 27814033.
- ^ Mariani, Patrizio; Andersen, Ken H.; Visser, André W.; Barton, Andrew D.; Kiørboe, Thomas (2013). "Control of plankton seasonal succession by adaptive grazing". Limnology and Oceanography. 58 (1): 173–184. Bibcode:2013LimOc..58..173M. doi:10.4319/lo.2013.58.1.0173.
- ^ Behrenfeld, Michael J. (2010). "Abandoning Sverdrup's Critical Depth Hypothesis on phytoplankton blooms". Ecology. 91 (4): 977–989. doi:10.1890/09-1207.1. PMID 20462113.
- ^ Mignot, A.; Ferrari, R.; Claustre, H. (2018). "Floats with bio-optical sensors reveal what processes trigger the North Atlantic bloom". Nature Communications. 9 (1): 190. Bibcode:2018NatCo...9..190M. doi:10.1038/s41467-017-02143-6. PMC 5768750. PMID 29335403.
- ^ Siegel, David A.; Buesseler, Ken O.; Behrenfeld, Michael J.; Benitez-Nelson, Claudia R.; Boss, Emmanuel; Brzezinski, Mark A.; Burd, Adrian; Carlson, Craig A.; d'Asaro, Eric A.; Doney, Scott C.; Perry, Mary J.; Stanley, Rachel H. R.; Steinberg, Deborah K. (2016). "Prediction of the Export and Fate of Global Ocean Net Primary Production: The EXPORTS Science Plan". Frontiers in Marine Science. 3. doi:10.3389/fmars.2016.00022.
 Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License.
Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License.
- ^ a b c d Steinberg, Deborah K.; Landry, Michael R. (2017). "Zooplankton and the Ocean Carbon Cycle". Annual Review of Marine Science. 9: 413–444. Bibcode:2017ARMS....9..413S. doi:10.1146/annurev-marine-010814-015924. PMID 27814033.
- ^ Saba, Grace K.; Steinberg, Deborah K.; Bronk, Deborah A. (2011). "The relative importance of sloppy feeding, excretion, and fecal pellet leaching in the release of dissolved carbon and nitrogen by Acartia tonsa copepods". Journal of Experimental Marine Biology and Ecology. 404 (1–2): 47–56. doi:10.1016/j.jembe.2011.04.013.
- ^ a b Thor, P.; Dam, HG; Rogers, DR (2003). "Fate of organic carbon released from decomposing copepod fecal pellets in relation to bacterial production and ectoenzymatic activity". Aquatic Microbial Ecology. 33: 279–288. doi:10.3354/ame033279.
- ^ Hansell, Dennis A.; Carlson, Craig A. (2 October 2014). Biogeochemistry of Marine Dissolved Organic Matter. ISBN 9780124071537.
- ^ Moller, E. F. (2004). "Sloppy feeding in marine copepods: Prey-size-dependent production of dissolved organic carbon". Journal of Plankton Research. 27: 27–35. doi:10.1093/plankt/fbh147.
- ^ Møller, Eva Friis (2007). "Production of dissolved organic carbon by sloppy feeding in the copepods Acartia tonsa, Centropages typicus, and Temora longicornis". Limnology and Oceanography. 52 (1): 79–84. Bibcode:2007LimOc..52...79M. doi:10.4319/lo.2007.52.1.0079.
- ^ a b c Halfter, Svenja; Cavan, Emma L.; Swadling, Kerrie M.; Eriksen, Ruth S.; Boyd, Philip W. (2020). "The Role of Zooplankton in Establishing Carbon Export Regimes in the Southern Ocean – A Comparison of Two Representative Case Studies in the Subantarctic Region". Frontiers in Marine Science. 7. doi:10.3389/fmars.2020.567917. S2CID 222003883.
 Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License.
Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License.
- ^ Møller, EF; Thor, P.; Nielsen, TG (2003). "Production of DOC by Calanus finmarchicus, C. Glacialis and C. Hyperboreus through sloppy feeding and leakage from fecal pellets". Marine Ecology Progress Series. 262: 185–191. Bibcode:2003MEPS..262..185M. doi:10.3354/meps262185.
- ^ Saba, GK; Steinberg, DK; Bronk, DA (2009). "Effects of diet on release of dissolved organic and inorganic nutrients by the copepod Acartia tonsa". Marine Ecology Progress Series. 386: 147–161. Bibcode:2009MEPS..386..147S. doi:10.3354/meps08070.
- ^ Doherty, S.; Maas, A. E.; Steinberg, D. K.; Popp, B. N.; Close, H. G. (2019). "Compound-Specific Isotope Analysis of Zooplankton Fecal Pellets: Insights into Dietary and Trophic Processes and Characterization of Fecal Pellets as Organic Matter End-Member". American Geophysical Union, Fall Meeting 2019 Abstracts. abstract #PP42C–12. Bibcode:2019AGUFMPP42C..12D.
External links
- SAHFOS Sir Alister Hardy Foundation for Ocean Science
- Ocean Drifters Short film narrated by David Attenborough about the varied roles of plankton
- Sea Drifters BBC Audio slideshow
- Plankton Chronicles Short documentary films & photos
- COPEPOD: The global plankton database. A global coverage database of zooplankton biomass and abundance data.
- Guide to the marine zooplankton of south eastern Australia, Tasmanian Aquaculture and Fisheries Institute
- Australian Continuous Plankton Recorder Project
- An Image-Based Key to Zooplankton of North America

![Upper left: Biogeochemical models Right: Ecosystem models Lower left: Size-spectra models These models also have temporal and spatial components.[4]](http://upload.wikimedia.org/wikipedia/commons/e/ec/Typical_ocean_models_featuring_zooplankton_2.jpg)






![The Egyptian pyramids were constructed from limestone that contained nummulites.[34]](http://upload.wikimedia.org/wikipedia/commons/thumb/a/af/All_Gizah_Pyramids.jpg/325px-All_Gizah_Pyramids.jpg)














![Oodinium, a genus of parasitic dinoflagellates, causes velvet disease in fish[40]](http://upload.wikimedia.org/wikipedia/commons/thumb/8/87/Archives_de_zoologie_exp%C3%A9rimentale_et_g%C3%A9n%C3%A9rale_%281920%29_%2820299351186%29.jpg/260px-Archives_de_zoologie_exp%C3%A9rimentale_et_g%C3%A9n%C3%A9rale_%281920%29_%2820299351186%29.jpg)
![Karenia brevis produces red tides highly toxic to humans[41]](http://upload.wikimedia.org/wikipedia/commons/thumb/a/a0/Karenia_brevis.jpg/214px-Karenia_brevis.jpg)

















![Pelagic food web and the biological pump. Links among the ocean's biological pump and pelagic food web and the ability to sample these components remotely from ships, satellites, and autonomous vehicles. Light blue waters are the euphotic zone, while the darker blue waters represent the twilight zone.[74]](http://upload.wikimedia.org/wikipedia/commons/thumb/1/13/Export_Processes_in_the_Ocean_from_Remote_Sensing.jpg/627px-Export_Processes_in_the_Ocean_from_Remote_Sensing.jpg)




