Circadian rhythm: Difference between revisions
Sherazade96 (talk | contribs) Added content at various places throughout including many citations and references. |
reformatted using diberri's tool |
||
| Line 7: | Line 7: | ||
== History == |
== History == |
||
The earliest known account of a circadian rhythm dates from the 4th century BC, when Androsthenes, a ship captain serving under [[Alexander the Great]], described [[Diurnality|diurnal]] leaf movements of the [[tamarind]] tree.<ref>Bretzl H. |
The earliest known account of a circadian rhythm dates from the 4th century BC, when Androsthenes, a ship captain serving under [[Alexander the Great]], described [[Diurnality|diurnal]] leaf movements of the [[tamarind]] tree.<ref>{{cite book |author=Bretzl H. |title=Botaniche Forchungen des Alexanderzuges |location=Leipzig |publisher=Teubner |year=1903}}{{pn}}</ref> The first modern observation of endogenous circadian oscillation was by the French scientist [[Jean-Jacques d'Ortous de Mairan]] in the 1700s; he noted that 24-hour patterns in the movement of the leaves of the plant ''[[Mimosa pudica]]'' continued even when the plants were isolated from external stimuli. |
||
In 1918, J. S. Szymanski showed that animals are capable of maintaining 24-hour activity patterns in the absence of external cues such as light and changes in temperature.<ref>{{cite journal | last = Danchin | first = Antoine | date = | year = | month = | title = Important dates 1900-1919 | journal = HKU-Pasteur Research Centre | publisher = | location = Paris | url = http://www.pasteur.fr/recherche/unites/REG/causeries/dates_1900.html | accessdate = 2008-01-12 | quote = }}</ref> [[Joseph Takahashi]] discovered the genetic basis for the rodent circadian rhythm in 1994.<ref>{{Cite news|title=Gene Discovered in Mice that Regulates Biological Clock |publisher=Chicago Tribune |date=April 29, 1994}}</ref><ref>{{cite journal| |
In 1918, J. S. Szymanski showed that animals are capable of maintaining 24-hour activity patterns in the absence of external cues such as light and changes in temperature.<ref>{{cite journal | last = Danchin | first = Antoine | date = | year = | month = | title = Important dates 1900-1919 | journal = HKU-Pasteur Research Centre | publisher = | location = Paris | url = http://www.pasteur.fr/recherche/unites/REG/causeries/dates_1900.html | accessdate = 2008-01-12 | quote = }}</ref> [[Joseph Takahashi]] discovered the genetic basis for the rodent circadian rhythm in 1994.<ref>{{Cite news|title=Gene Discovered in Mice that Regulates Biological Clock |publisher=Chicago Tribune |date=April 29, 1994}}</ref><ref>{{cite journal |author=Vitaterna MH, King DP, Chang AM, ''et al.'' |title=Mutagenesis and mapping of a mouse gene, Clock, essential for circadian behavior |journal=Science |volume=264 |issue=5159 |pages=719–25 |year=1994 |month=April |pmid=8171325 |doi=10.1126/science.8171325}}</ref> |
||
== Criteria == |
== Criteria == |
||
To differentiate genuinely endogenous circadian rhythms from coincidental or apparent ones, three general criteria must be met: 1) the rhythms persist in the absence of cues, 2) they persist equally precisely over a range of temperatures, and 3) the rhythms can be adjusted to match the local time: |
To differentiate genuinely endogenous circadian rhythms from coincidental or apparent ones, three general criteria must be met: 1) the rhythms persist in the absence of cues, 2) they persist equally precisely over a range of temperatures, and 3) the rhythms can be adjusted to match the local time: |
||
* The rhythm persists in constant conditions (for example, constant dark) with a period of about 24 hours. The rationale for this criterion is to distinguish circadian rhythms from those "apparent" rhythms that are merely responses to external periodic cues. A rhythm cannot be declared to be endogenous unless it has been tested in conditions without external periodic input. |
* The rhythm persists in constant conditions (for example, constant dark) with a period of about 24 hours. The rationale for this criterion is to distinguish circadian rhythms from those "apparent" rhythms that are merely responses to external periodic cues. A rhythm cannot be declared to be endogenous unless it has been tested in conditions without external periodic input. |
||
* The rhythm is temperature-compensated, i.e., it maintains the same period over a range of temperatures. The rationale for this criterion is to distinguish circadian rhythms from other biological rhythms arising due to the circular nature of a reaction pathway. At a low enough or high enough temperature, the period of a circular reaction may reach 24 hours, but it will be merely coincidental. |
* The rhythm is temperature-compensated, i.e., it maintains the same period over a range of temperatures. The rationale for this criterion is to distinguish circadian rhythms from other biological rhythms arising due to the circular nature of a reaction pathway. At a low enough or high enough temperature, the period of a circular reaction may reach 24 hours, but it will be merely coincidental. |
||
| Line 24: | Line 22: | ||
Photosensitive proteins and circadian rhythms are believed to have originated in the earliest cells, with the purpose of protecting the replicating of DNA from high [[ultraviolet]] radiation during the daytime. As a result, replication was relegated to the dark. The fungus ''[[Neurospora]]'', which exists today, retains this [[Circadian Oscillator|clock-regulated mechanism]]. |
Photosensitive proteins and circadian rhythms are believed to have originated in the earliest cells, with the purpose of protecting the replicating of DNA from high [[ultraviolet]] radiation during the daytime. As a result, replication was relegated to the dark. The fungus ''[[Neurospora]]'', which exists today, retains this [[Circadian Oscillator|clock-regulated mechanism]]. |
||
Circadian rhythms allow organisms to anticipate and prepare for precise and regular environmental changes; they have great value in relation to the outside world. The rhythmicity appears to be as important in regulating and coordinating internal metabolic processes, as in coordinating with the environment.<ref>{{cite journal | |
Circadian rhythms allow organisms to anticipate and prepare for precise and regular environmental changes; they have great value in relation to the outside world. The rhythmicity appears to be as important in regulating and coordinating internal metabolic processes, as in coordinating with the environment.<ref>{{cite journal |author=Sharma VK |title=Adaptive significance of circadian clocks |journal=Chronobiology International |volume=20 |issue=6 |pages=901–19 |year=2003 |month=November |pmid=14680135 |doi=10.1081/CBI-120026099}}</ref> This is suggested by the maintenance (heritability) of circadian rhythms in fruit flies after several hundred generations in constant laboratory conditions,<ref>(Sheeba et al. 1999)</ref> as well as in creatures in constant darkness in the wild, and by the experimental elimination of behavioural but not physiological circadian rhythms in quail.<ref>Guyomarc'h et al. 1998{{vs|need more bibliographic info}}</ref><ref>Zivkovic et al. 1999{{vs|need more bibliographic info}}</ref> |
||
The simplest known circadian clock is that of the prokaryotic [[cyanobacteria]]. Recent research has demonstrated that the circadian clock of ''Synechococcus elongatus'' can be reconstituted ''in vitro'' with just the three proteins of their central oscillator. This clock has been shown to sustain a 22-hour rhythm over several days upon the addition of [[Adenosine triphosphate|ATP]]. Previous explanations of the [[prokaryotic]] circadian timekeeper were dependent upon a DNA transcription / translation feedback mechanism. |
The simplest known circadian clock is that of the prokaryotic [[cyanobacteria]]. Recent research has demonstrated that the circadian clock of ''Synechococcus elongatus'' can be reconstituted ''in vitro'' with just the three proteins of their central oscillator. This clock has been shown to sustain a 22-hour rhythm over several days upon the addition of [[Adenosine triphosphate|ATP]]. Previous explanations of the [[prokaryotic]] circadian timekeeper were dependent upon a DNA transcription / translation feedback mechanism. |
||
| Line 32: | Line 30: | ||
In 1971, Ronald J. Konopka and [[Seymour Benzer]] first identified a genetic component of the biological clock using the fruit fly as a model system. Three mutant lines of flies displayed aberrant behaviour: one had a shorter period, another had a longer one, and the third had none. All three mutations mapped to the same gene, which was named ''[[period (gene)|period]]''.<ref>{{cite book |last= Purves |first= Dale ''et al'' |title= NEUROSCIENCE |origdate= |origyear= 2001 |url= http://www.ncbi.nlm.nih.gov/books/bv.fcgi?rid=.0-X5CQEZnQIQrizQvUwzgMbTAG |format= e-book |accessdate= 2008-05-30 |edition= second |series= |date= |year= 2001|month= |publisher= Sinauer Associates |location= Sunderland, MA, U.S.A. |isbn= 0-87893-742-0 |oclc= |doi= |id= |pages= |chapter= Molecular Mechanisms of Biological Clocks |chapterurl= http://www.ncbi.nlm.nih.gov/books/bv.fcgi?rid=neurosci.box.1963 |quote= }}</ref> The same gene was identified to be defective in the sleep disorder FASPS ([[Familial advanced sleep phase syndrome]]) in human beings thirty years later, underscoring the conserved nature of the molecular circadian clock through evolution. Many more genetic components of the biological clock are now known. Their interactions result in an interlocked feedback loop of gene products resulting in periodic fluctuations that the cells of the body interpret as a specific time of the day. |
In 1971, Ronald J. Konopka and [[Seymour Benzer]] first identified a genetic component of the biological clock using the fruit fly as a model system. Three mutant lines of flies displayed aberrant behaviour: one had a shorter period, another had a longer one, and the third had none. All three mutations mapped to the same gene, which was named ''[[period (gene)|period]]''.<ref>{{cite book |last= Purves |first= Dale ''et al'' |title= NEUROSCIENCE |origdate= |origyear= 2001 |url= http://www.ncbi.nlm.nih.gov/books/bv.fcgi?rid=.0-X5CQEZnQIQrizQvUwzgMbTAG |format= e-book |accessdate= 2008-05-30 |edition= second |series= |date= |year= 2001|month= |publisher= Sinauer Associates |location= Sunderland, MA, U.S.A. |isbn= 0-87893-742-0 |oclc= |doi= |id= |pages= |chapter= Molecular Mechanisms of Biological Clocks |chapterurl= http://www.ncbi.nlm.nih.gov/books/bv.fcgi?rid=neurosci.box.1963 |quote= }}</ref> The same gene was identified to be defective in the sleep disorder FASPS ([[Familial advanced sleep phase syndrome]]) in human beings thirty years later, underscoring the conserved nature of the molecular circadian clock through evolution. Many more genetic components of the biological clock are now known. Their interactions result in an interlocked feedback loop of gene products resulting in periodic fluctuations that the cells of the body interpret as a specific time of the day. |
||
A great deal of research on biological clocks was done in the latter half of the 20th century. It is now known that the molecular circadian clock can function within a single cell; i.e., it is cell-autonomous.<ref> |
A great deal of research on biological clocks was done in the latter half of the 20th century. It is now known that the molecular circadian clock can function within a single cell; i.e., it is cell-autonomous.<ref>{{cite journal |author=Nagoshi E, Saini C, Bauer C, Laroche T, Naef F, Schibler U |title=Circadian gene expression in individual fibroblasts: cell-autonomous and self-sustained oscillators pass time to daughter cells |journal=Cell |volume=119 |issue=5 |pages=693–705 |year=2004 |month=November |pmid=15550250 |doi=10.1016/j.cell.2004.11.015}}</ref> At the same time, different cells may communicate with each other resulting in a synchronized output of electrical signaling. These may interface with endocrine glands of the brain to result in periodic release of hormones. The receptors for these hormones may be located far across the body and synchronize the peripheral clocks of various organs. Thus, the information of the time of the day as relayed by the [[eye]]s travels to the clock in the brain, and, through that, clocks in the rest of the body may be synchronized. This is how the timing of, for example, sleep/wake, body temperature, thirst, and appetite are coordinately controlled by the biological clock. |
||
== Importance in animals == |
== Importance in animals == |
||
Circadian rhythmicity is present in the [[sleep]]ing and feeding patterns of animals, including human beings. There are also clear patterns of core body temperature, [[brain wave]] activity, [[hormone]] production, cell regeneration and other biological activities. In addition, [[photoperiodism]], the physiological reaction of organisms to the length of day or night, is vital to both plants and animals, and the circadian system plays a role in the measurement and interpretation of day length. |
Circadian rhythmicity is present in the [[sleep]]ing and feeding patterns of animals, including human beings. There are also clear patterns of core body temperature, [[brain wave]] activity, [[hormone]] production, cell regeneration and other biological activities. In addition, [[photoperiodism]], the physiological reaction of organisms to the length of day or night, is vital to both plants and animals, and the circadian system plays a role in the measurement and interpretation of day length. |
||
{{cquote|Timely prediction of seasonal periods of weather conditions, food availability or predator activity is crucial for survival of many species. Although not the only parameter, the changing length of the photoperiod ('daylength') is the most predictive environmental cue for the seasonal timing of physiology and behavior, most notably for timing of migration, hibernation and reproduction.<ref>{{cite web |url= http://scienceblogs.com/clock/2007/07/clock_tutorial_16_photoperiodi_1.php |title=Clock Tutorial #16: Photoperiodism - Models and Experimental Approaches |accessdate=2007-12-09 |last=Zivkovic |first=Bora "Coturnix" |date=2005-08-13 / July 25, 2007 |work=A Blog Around the Clock |publisher=ScienceBlogs |quote= }}</ref>}} |
{{cquote|Timely prediction of seasonal periods of weather conditions, food availability or predator activity is crucial for survival of many species. Although not the only parameter, the changing length of the photoperiod ('daylength') is the most predictive environmental cue for the seasonal timing of physiology and behavior, most notably for timing of migration, hibernation and reproduction.<ref>{{cite web |url= http://scienceblogs.com/clock/2007/07/clock_tutorial_16_photoperiodi_1.php |title=Clock Tutorial #16: Photoperiodism - Models and Experimental Approaches |accessdate=2007-12-09 |last=Zivkovic |first=Bora "Coturnix" |date=2005-08-13 / July 25, 2007 |work=A Blog Around the Clock |publisher=ScienceBlogs |quote= }}</ref>}} |
||
=== Impact of light–dark cycle === |
=== Impact of light–dark cycle === |
||
The rhythm is linked to the light–dark cycle. Animals, including humans, kept in total darkness for extended periods eventually function with a [[Free-running sleep|freerunning]] rhythm. Each "day", their sleep cycle is pushed back or forward, depending on whether their [[endogenous]] period is shorter or longer than 24 hours. The environmental cues that each day reset the rhythms are called ''[[Zeitgeber]]s'' (from the German, ''Time Givers'').<ref name=health.am>{{cite web | Shneerson JM, Ohayon MM, Carskadon MA | title =Circadian rhythms | publisher=Armenian Medical Network | work = Rapid eye movement (REM) sleep | url=http://www.sleep.health.am/sleep/more/circadian-rhythms/ | year = 2007 | accessdate=2007-09-19}}</ref> It is interesting to note that totally-blind subterranean mammals (e.g., [[blind mole rat]] ''Spalax'' sp.) are able to maintain their endogenous clocks in the apparent absence of external stimuli. Although they lack image-forming eyes, their photoreceptors (detect light) are still functional; as well, they do surface periodically.{{Citation needed|date=September 2009}} |
The rhythm is linked to the light–dark cycle. Animals, including humans, kept in total darkness for extended periods eventually function with a [[Free-running sleep|freerunning]] rhythm. Each "day", their sleep cycle is pushed back or forward, depending on whether their [[endogenous]] period is shorter or longer than 24 hours. The environmental cues that each day reset the rhythms are called ''[[Zeitgeber]]s'' (from the German, ''Time Givers'').<ref name=health.am>{{cite web | Shneerson JM, Ohayon MM, Carskadon MA | title =Circadian rhythms | publisher=Armenian Medical Network | work = Rapid eye movement (REM) sleep | url=http://www.sleep.health.am/sleep/more/circadian-rhythms/ | year = 2007 | accessdate=2007-09-19}}</ref> It is interesting to note that totally-blind subterranean mammals (e.g., [[blind mole rat]] ''Spalax'' sp.) are able to maintain their endogenous clocks in the apparent absence of external stimuli. Although they lack image-forming eyes, their photoreceptors (detect light) are still functional; as well, they do surface periodically.{{Citation needed|date=September 2009}} |
||
| Line 48: | Line 44: | ||
=== Arctic animals === |
=== Arctic animals === |
||
Norwegian researchers at the [[University of Tromsø]] have shown that some Arctic animals ([[ptarmigan]], [[reindeer]]) show circadian rhythms only in the parts of the year that have daily sunrises and sunsets. In one study of reindeer, animals at [[70th parallel north|70 degrees North]] showed circadian rhythms in the autumn, winter, and spring, but not in the summer. Reindeer at [[78th parallel north|78 degrees North]] showed such rhythms only autumn and spring. The researchers suspect that other Arctic animals as well may not show circadian rhythms in the constant light of summer and the constant dark of winter.<ref>{{cite news |first=Ingrid |last=Spilde |author= |title=Reinsdyr uten døgnrytme |url= http://www.forskning.no/Artikler/2005/desember/1135264557.29 |format= |work= |publisher=forskning.no |date=December 2005 |accessdate=2007-11-24 |language=Language: Norwegian, Bokmål |quote= |archiveurl= |archivedate= }}</ref><ref>{{cite web |url=http://scienceblogs.com/clock/2007/07/circadian_rhythms_or_not_in_ar_1.php |
Norwegian researchers at the [[University of Tromsø]] have shown that some Arctic animals ([[ptarmigan]], [[reindeer]]) show circadian rhythms only in the parts of the year that have daily sunrises and sunsets. In one study of reindeer, animals at [[70th parallel north|70 degrees North]] showed circadian rhythms in the autumn, winter, and spring, but not in the summer. Reindeer at [[78th parallel north|78 degrees North]] showed such rhythms only autumn and spring. The researchers suspect that other Arctic animals as well may not show circadian rhythms in the constant light of summer and the constant dark of winter.<ref>{{cite news |first=Ingrid |last=Spilde |author= |title=Reinsdyr uten døgnrytme |url= http://www.forskning.no/Artikler/2005/desember/1135264557.29 |format= |work= |publisher=forskning.no |date=December 2005 |accessdate=2007-11-24 |language=Language: Norwegian, Bokmål |quote= |archiveurl= |archivedate= }}</ref><ref>{{cite web |url=http://scienceblogs.com/clock/2007/07/circadian_rhythms_or_not_in_ar_1.php |
||
|title=Circadian Rhythms, or Not, in Arctic Reindeer |accessdate=2007-11-24 |author= |last=Zivkovic |first=Bora, aka Coturnix, chronobiologist |authorlink= |date= |year= |month= |format= |work=A Blog around the Clock |publisher=ScienceBlogs.com }}</ref> |
|title=Circadian Rhythms, or Not, in Arctic Reindeer |accessdate=2007-11-24 |author= |last=Zivkovic |first=Bora, aka Coturnix, chronobiologist |authorlink= |date= |year= |month= |format= |work=A Blog around the Clock |publisher=ScienceBlogs.com }}</ref> |
||
| Line 57: | Line 52: | ||
=== Butterfly migration === |
=== Butterfly migration === |
||
| ⚫ | The navigation of the fall migration of the [[Monarch (butterfly)|Eastern North American monarch butterfly]] (''Danaus plexippus'') to their overwintering grounds in central Mexico uses a time-compensated sun compass that depends upon a circadian clock in their antennae.<ref>{{cite journal |author=Merlin C, Gegear RJ, Reppert SM |title=Antennal circadian clocks coordinate sun compass orientation in migratory monarch butterflies |journal=Science |volume=325 |issue=5948 |pages=1700–4 |year=2009 |month=September |pmid=19779201 |doi=10.1126/science.1176221}}</ref><ref>{{cite journal |author=Kyriacou CP |title=Physiology. Unraveling traveling |journal=Science |volume=325 |issue=5948 |pages=1629–30 |year=2009 |month=September |pmid=19779177 |doi=10.1126/science.1178935}}</ref> |
||
| ⚫ | The navigation of the fall migration of the [[Monarch (butterfly)|Eastern North American monarch butterfly]] (''Danaus plexippus'') to their overwintering grounds in central Mexico uses a time-compensated sun compass that depends upon a circadian clock in their antennae.<ref>Merlin C, Gegear RJ, Reppert SM |
||
== Biological clock in mammals == |
== Biological clock in mammals == |
||
| Line 69: | Line 63: | ||
The SCN takes the information on the lengths of the day and night from the retina, interprets it, and passes it on to the [[pineal gland]], a tiny structure shaped like a [[pine cone]] and located on the [[epithalamus]]. In response the pineal secretes the hormone [[melatonin]]. Secretion of melatonin peaks at night and ebbs during the day and its presence provides information about night-length. |
The SCN takes the information on the lengths of the day and night from the retina, interprets it, and passes it on to the [[pineal gland]], a tiny structure shaped like a [[pine cone]] and located on the [[epithalamus]]. In response the pineal secretes the hormone [[melatonin]]. Secretion of melatonin peaks at night and ebbs during the day and its presence provides information about night-length. |
||
The circadian rhythms of humans can be entrained to slightly shorter and longer periods than the Earth's 24 hours. Researchers at Harvard have recently shown that human subjects can at least be entrained to a 23.5-hour cycle and a 24.65-hour cycle (the latter being the natural solar day-night cycle on the planet [[Mars]]).<ref>{{cite journal | |
The circadian rhythms of humans can be entrained to slightly shorter and longer periods than the Earth's 24 hours. Researchers at Harvard have recently shown that human subjects can at least be entrained to a 23.5-hour cycle and a 24.65-hour cycle (the latter being the natural solar day-night cycle on the planet [[Mars]]).<ref>{{cite journal |author=Scheer FA, Wright KP, Kronauer RE, Czeisler CA |title=Plasticity of the intrinsic period of the human circadian timing system |journal=PLos ONE |volume=2 |issue=1 |pages=e721 |year=2007 |pmid=17684566 |pmc=1934931 |doi=10.1371/journal.pone.0000721}}</ref> |
||
=== Determining the human circadian rhythm === |
=== Determining the human circadian rhythm === |
||
The classic phase markers for measuring the timing of a mammal's circadian rhythm are<ref name="phasemarkers">{{ |
The classic phase markers for measuring the timing of a mammal's circadian rhythm are<ref name="phasemarkers">{{cite journal |author=Benloucif S, Guico MJ, Reid KJ, Wolfe LF, L'hermite-Balériaux M, Zee PC |title=Stability of melatonin and temperature as circadian phase markers and their relation to sleep times in humans |journal=Journal of Biological Rhythms |volume=20 |issue=2 |pages=178–88 |year=2005 |month=April |pmid=15834114 |doi=10.1177/0748730404273983}}</ref> |
||
| url= http://jbr.sagepub.com/cgi/content/abstract/20/2/178 |
|||
| title=Stability of Melatonin and Temperature as Circadian Phase Markers and Their Relation to Sleep Times in Humans |
|||
| journal=Journal of Biological Rhythms |
|||
| last = Benloucif |
|||
| first = S. |
|||
| coauthors = Guico, M.J.; Reid, K.J.; Wolfe, L.F.; L'Hermite-Baleriaux, M.; Zee, P.C. |
|||
| publisher=SAGE Publications |
|||
| location = Chicago, Illinois, USA |
|||
| year=2005 |
|||
| volume=20 |
|||
| issue=2 |
|||
| pages=178-188 |
|||
| issn=0748-7304 |
|||
| doi=10.1177/0748730404273983 |
|||
| pmid=15834114 |
|||
| accessdate=2010-01-27 |
|||
}}</ref><!-- this ref has online ISSN of 1552-4531 --> |
|||
*melatonin secretion by the pineal gland and |
*melatonin secretion by the pineal gland and |
||
| Line 105: | Line 82: | ||
=== Outside the "master clock" === |
=== Outside the "master clock" === |
||
| ⚫ | More-or-less independent circadian rhythms are found in many organs and cells in the body outside the suprachiasmatic nuclei (SCN), the "master clock". These clocks, called peripheral oscillators, are found in the [[esophagus|oesophagus]], [[lungs]], [[liver]], [[pancreas]], [[spleen]], [[thymus]], and the [[skin]].<ref>{{cite journal |author=Zanello SB, Jackson DM, Holick MF |title=Expression of the circadian clock genes clock and period1 in human skin |journal=The Journal of Investigative Dermatology |volume=115 |issue=4 |pages=757–60 |year=2000 |month=October |pmid=10998156 |doi=10.1046/j.1523-1747.2000.00121.x}}</ref> Though oscillators in the skin respond to light, a systemic influence has not been proven so far.<ref>{{cite journal |author=Kawara S, Mydlarski R, Mamelak AJ, ''et al.'' |title=Low-dose ultraviolet B rays alter the mRNA expression of the circadian clock genes in cultured human keratinocytes |journal=The Journal of Investigative Dermatology |volume=119 |issue=6 |pages=1220–3 |year=2002 |month=December |pmid=12485420 |doi=10.1046/j.1523-1747.2002.19619.x}}</ref><ref>{{cite journal |author=Campbell SS, Murphy PJ |title=Extraocular circadian phototransduction in humans |journal=Science |volume=279 |issue=5349 |pages=396–9 |year=1998 |month=January |pmid=9430592 |doi=10.1126/science.279.5349.396}}</ref> There is some evidence that also the olfactory bulb and prostate may experience oscillations when cultured, suggesting that also these structures may be weak oscillators. |
||
| ⚫ | More-or-less independent circadian rhythms are found in many organs and cells in the body outside the suprachiasmatic nuclei (SCN), the "master clock". These clocks, called peripheral oscillators, are found in the [[esophagus|oesophagus]], [[lungs]], [[liver]], [[pancreas]], [[spleen]], [[thymus]], and the [[skin]].<ref> |
||
Furthermore, liver cells, for example, appear to respond to feeding rather than to [[light]]. Cells from many parts of the body appear to have freerunning rhythms. |
Furthermore, liver cells, for example, appear to respond to feeding rather than to [[light]]. Cells from many parts of the body appear to have freerunning rhythms. |
||
== Light and the biological clock == |
== Light and the biological clock == |
||
Light resets the biological clock in accordance with the [[phase response curve]] (PRC). Depending on the timing, light can advance or delay the circadian rhythm. Both the PRC and the required [[illuminance]] vary from species to species and lower light levels are required to reset the clocks in nocturnal rodents than in humans. |
Light resets the biological clock in accordance with the [[phase response curve]] (PRC). Depending on the timing, light can advance or delay the circadian rhythm. Both the PRC and the required [[illuminance]] vary from species to species and lower light levels are required to reset the clocks in nocturnal rodents than in humans. |
||
Lighting levels that affect circadian rhythm in humans are higher than the levels usually used in artificial lighting in homes. According to some researchers<ref name=semj /> the illumination intensity that excites the circadian system has to reach up to 1000 [[lux]] striking the retina. In addition to light intensity, wavelength (or colour) of light is a factor in the entrainment of the body clock. [[Melanopsin]] is most efficiently excited by blue light, 420–440 nm<ref>Newman LA, Walker MT, Brown RL, Cronin TW, Robinson PR |
Lighting levels that affect circadian rhythm in humans are higher than the levels usually used in artificial lighting in homes. According to some researchers<ref name=semj /> the illumination intensity that excites the circadian system has to reach up to 1000 [[lux]] striking the retina. In addition to light intensity, wavelength (or colour) of light is a factor in the entrainment of the body clock. [[Melanopsin]] is most efficiently excited by blue light, 420–440 nm<ref>{{cite journal |author=Newman LA, Walker MT, Brown RL, Cronin TW, Robinson PR |title=Melanopsin forms a functional short-wavelength photopigment |journal=Biochemistry |volume=42 |issue=44 |pages=12734–8 |year=2003 |month=November |pmid=14596587 |doi=10.1021/bi035418z}}</ref> according to some researchers while others have reported 470–485 nm. |
||
It is thought that the direction of the light may have an effect on entraining the circadian rhythm;<ref name=semj>{{cite web | last=Semjonova | first=Milena | title =Healthy Lighting, from a lighting designer's perspective | url= http://www.enlighter.org/images/2009/01/healthyLighting.pdf | year = 2003 | publisher= Milena Lighting Design }}</ref> light coming from above, resembling an image of a bright sky, has greater effect than light entering our eyes from below. |
It is thought that the direction of the light may have an effect on entraining the circadian rhythm;<ref name=semj>{{cite web | last=Semjonova | first=Milena | title =Healthy Lighting, from a lighting designer's perspective | url= http://www.enlighter.org/images/2009/01/healthyLighting.pdf | year = 2003 | publisher= Milena Lighting Design }}</ref> light coming from above, resembling an image of a bright sky, has greater effect than light entering our eyes from below. |
||
According to a 2010 study completed by the Lighting Research Center |
According to a 2010 study completed by the Lighting Research Center, daylight has a direct effect on circadian rhythms and, consequently, on performance and well-being. The research showed that students who experience disruption in lighting schemes in the morning consequently experience disruption in sleeping patterns. The change in sleeping patterns may lead to negatively impacted student performance and alertness. Removing circadian light in the morning delays the dim light melatonin onset by 6 minutes a day, for a total of 30 minutes for five days.<ref>{{cite journal |author=Figueiro MG, Rea MS |title=Lack of short-wavelength light during the school day delays dim light melatonin onset (DLMO) in middle school students |journal=Neuro Endocrinology Letters |volume=31 |issue=1 |pages=4 |year=2010 |month=February |pmid=20150866}}</ref> |
||
== Enforced longer cycles == |
== Enforced longer cycles == |
||
Modern research under very controlled conditions has shown the human period for adults to be just slightly longer than 24 hours on average. Czeisler ''et al.'' at Harvard found the range for normal, healthy adults of all ages to be quite narrow: 24 hours and 11 minutes ± 16 minutes. The "clock" resets itself daily to the 24-hour cycle of the Earth's rotation.<ref name=hno>{{cite web | Charles A. Czeisler MD, PhD | title =Human Biological Clock Set Back an Hour | url=http://www.hno.harvard.edu/gazette/1999/07.15/bioclock24.html | year = 1999 | accessdate=2007-09-23 | quote= The variation between our subjects, with a 95 percent level of confidence, was no more than plus or minus 16 minutes, a remarkably small range. }}</ref> |
Modern research under very controlled conditions has shown the human period for adults to be just slightly longer than 24 hours on average. Czeisler ''et al.'' at Harvard found the range for normal, healthy adults of all ages to be quite narrow: 24 hours and 11 minutes ± 16 minutes. The "clock" resets itself daily to the 24-hour cycle of the Earth's rotation.<ref name=hno>{{cite web | Charles A. Czeisler MD, PhD | title =Human Biological Clock Set Back an Hour | url=http://www.hno.harvard.edu/gazette/1999/07.15/bioclock24.html | year = 1999 | accessdate=2007-09-23 | quote= The variation between our subjects, with a 95 percent level of confidence, was no more than plus or minus 16 minutes, a remarkably small range. }}</ref> |
||
The ''28-hour day'' is presented as a concept of [[time management]].<ref>{{cite web |url= http://www.dbeat.com/28/benefit2.htm |title= 28 Hour Day |accessdate= 2008-02-19 |author= Digital Beat Productions |year= 1997 |work= |publisher= |quote= }}</ref> It builds on the fact that the week of seven days at 24 hours and a "week" of six days at 28 hours both equal a week of 168 hours. To live on the 28-hour day and six-day week would require staying awake for 19 to 20 hours and sleeping for eight to nine hours. Each "day" on this system has a unique light/dark pattern. |
The ''28-hour day'' is presented as a concept of [[time management]].<ref>{{cite web |url= http://www.dbeat.com/28/benefit2.htm |title= 28 Hour Day |accessdate= 2008-02-19 |author= Digital Beat Productions |year= 1997 |work= |publisher= |quote= }}</ref> It builds on the fact that the week of seven days at 24 hours and a "week" of six days at 28 hours both equal a week of 168 hours. To live on the 28-hour day and six-day week would require staying awake for 19 to 20 hours and sleeping for eight to nine hours. Each "day" on this system has a unique light/dark pattern. |
||
Studies by [[Nathaniel Kleitman]]<ref>{{cite book|last=Kleitman |first=Nathaniel |title=Sleep and Wakefullness ed 2|location=Chicago|publisher= University of Chicago Press|year=1962}}</ref> in 1938 and by [[Derk-Jan Dijk]] and [[Charles Czeisler]]<ref>{{cite journal| |
Studies by [[Nathaniel Kleitman]]<ref>{{cite book|last=Kleitman |first=Nathaniel |title=Sleep and Wakefullness ed 2|location=Chicago|publisher= University of Chicago Press|year=1962}}</ref> in 1938 and by [[Derk-Jan Dijk]] and [[Charles Czeisler]]<ref>{{cite journal |author=Dijk DJ, Czeisler CA |title=Paradoxical timing of the circadian rhythm of sleep propensity serves to consolidate sleep and wakefulness in humans |journal=Neuroscience Letters |volume=166 |issue=1 |pages=63–8 |year=1994 |month=January |pmid=8190360 |doi=10.1016/0304-3940(94)90841-9}}</ref><ref>{{cite journal |author=Dijk DJ, Czeisler CA |title=Contribution of the circadian pacemaker and the sleep homeostat to sleep propensity, sleep structure, electroencephalographic slow waves, and sleep spindle activity in humans |journal=The Journal of Neuroscience |volume=15 |issue=5 Pt 1 |pages=3526–38 |year=1995 |month=May |pmid=7751928 |url=http://www.jneurosci.org/cgi/pmidlookup?view=long&pmid=7751928}}</ref> in 1994/5 have put human subjects on enforced 28-hour sleep–wake cycles, in constant dim light and with other time cues suppressed, for over a month. Because normal people cannot entrain to a 28-hour day,<ref>{{cite web |url= http://www.hno.harvard.edu/gazette/1999/07.15/bioclock24.html |title= Human Biological Clock Set Back an Hour |accessdate= 2008-02-19 |author= |last= Cromie |first= William J. |date= 1999-07-15 |work= |publisher= The Harvard University Gazette |quote= }}</ref> this is referred to as a forced desynchrony protocol. Sleep and wake episodes are uncoupled from the endogenous circadian period of about 24.18 hours and researchers are allowed to assess the effects of circadian phase on aspects of sleep and wakefulness including [[sleep latency]] and other functions.<ref>{{cite book | last = Aldrich | first = Michael S | title = Sleep medicine | year = 1999 | url = http://books.google.com.au/books?id=1jScwMrsmAMC&pg=RA1-PA65&lpg=RA1-PA65&dq=experimenting+with+the+28+hour+day&source=bl&ots=9R4mo2fI1O&sig=om2zbYPnXnm_1HuZo2Tch6J1vyo&hl=en&ei=MBZeStGgIoyJkQWd17znDA&sa=X&oi=book_result&ct=result&resnum=2 | isbn = 0-19-512957-1 | publisher = Oxford University Press | location = New York}}</ref> |
||
Early research into circadian rhythms suggested that most people preferred a day closer to 25 hours when isolated from external stimuli like daylight and timekeeping. Early investigators determined the human circadian period to be 25 hours or more. They went to great lengths to shield subjects from time cues and daylight, but they were not aware of the effects of indoor electric lights. The subjects were allowed to turn on light when they were awake and to turn it off when they wanted to sleep. Electric light in the evening delayed their circadian phase. These results became well known.<ref name=hno /> Researchers allowed subjects to keep electric lighting on in the evening, as it was thought at that time that a couple of 60W bulbs would not have a resetting effect on the circadian rhythms of humans. More recent research{{Citation needed|date=December 2009}} has shown that adults have a built-in day, which averages just over 24 hours, that indoor lighting does affect circadian rhythms and that most people attain their best-quality sleep during their [[chronotype]]-determined sleep periods. |
Early research into circadian rhythms suggested that most people preferred a day closer to 25 hours when isolated from external stimuli like daylight and timekeeping. Early investigators determined the human circadian period to be 25 hours or more. They went to great lengths to shield subjects from time cues and daylight, but they were not aware of the effects of indoor electric lights. The subjects were allowed to turn on light when they were awake and to turn it off when they wanted to sleep. Electric light in the evening delayed their circadian phase. These results became well known.<ref name=hno /> Researchers allowed subjects to keep electric lighting on in the evening, as it was thought at that time that a couple of 60W bulbs would not have a resetting effect on the circadian rhythms of humans. More recent research{{Citation needed|date=December 2009}} has shown that adults have a built-in day, which averages just over 24 hours, that indoor lighting does affect circadian rhythms and that most people attain their best-quality sleep during their [[chronotype]]-determined sleep periods. |
||
== Human health == |
== Human health == |
||
Timing of medical treatment in coordination with the body clock may significantly increase efficacy and reduce drug toxicity or adverse reactions. For example, appropriately timed treatment with [[angiotensin converting enzyme inhibitors]] (ACEi) may reduce nocturnal blood pressure and also benefit [[left ventricular]] (reverse) remodelling. {{Citation needed|date=January 2009}} |
Timing of medical treatment in coordination with the body clock may significantly increase efficacy and reduce drug toxicity or adverse reactions. For example, appropriately timed treatment with [[angiotensin converting enzyme inhibitors]] (ACEi) may reduce nocturnal blood pressure and also benefit [[left ventricular]] (reverse) remodelling. {{Citation needed|date=January 2009}} |
||
[[File:Day Sleepers crop.jpg|thumb|right|A short nap during the day does not affect circadian rhythms.]] |
[[File:Day Sleepers crop.jpg|thumb|right|A short nap during the day does not affect circadian rhythms.]] |
||
A number of studies have concluded that a short period of sleep during the day, a [[power-nap]], does not have any effect on normal circadian rhythm, but can decrease stress and improve productivity.<ref name=INST>{{cite |
A number of studies have concluded that a short period of sleep during the day, a [[power-nap]], does not have any effect on normal circadian rhythm, but can decrease stress and improve productivity.<ref name=INST>{{cite journal |author=Pilcher JJ, Michalowski KR, Carrigan RD |title=The prevalence of daytime napping and its relationship to nighttime sleep |journal=Behavioral Medicine |volume=27 |issue=2 |pages=71–6 |year=2001 |pmid=11763827 |url=http://heldref.metapress.com/openurl.asp?genre=article&issn=0896-4289&volume=27&issue=2&spage=71}}</ref><ref name=AAHPERD>{{cite web |Emily Rolston, Judy R. Sandlin, Michael Sandlin, and Rosanne Keathley | title=Power-Napping: Effects on Cognitive Ability and Stress Levels Among College Students | publisher=Liberty University | work=Power-Napping: Effects on Cognitive Ability and Stress Levels Among College Students | url=http://aahperd.confex.com/aahperd/2007/finalprogram/paper_10353.htm | year=2007 | accessdate=2008-11-11}}</ref> |
||
There are many health problems associated with disturbances of the human circadian rhythm, such as [[seasonal affective disorder]] (SAD), [[delayed sleep phase syndrome]] (DSPS) and other [[circadian rhythm disorder]]s.<ref name=brynmawr>{{cite web | Sabah Quraishi | title =Circadian Rhythms and Sleep | publisher=Serendip | work = Circadian Rhythms and Sleep | url=http://serendip.brynmawr.edu/bb/neuro/neuro01/web1/Quirashi.html | year = 2007 | accessdate=2007-09-19}}</ref> Circadian rhythms also play a part in the [[reticular activating system]], which is crucial for maintaining a state of consciousness. In addition, a reversal in the sleep–wake cycle may be a sign or complication of [[uremia]],<ref>{{cite web |url= http://www.emedicine.com/emerg/topic500.htm |title= Renal Failure, Acute |accessdate= 2008-08-03 |last= Sinert |first= Richard |coauthors= Peter R Peacock, Jr |date= May 10, 2006 |work= |publisher= eMedicine from WebMD |doi= |quote= }}</ref> [[azotemia]] or [[acute renal failure]]. |
There are many health problems associated with disturbances of the human circadian rhythm, such as [[seasonal affective disorder]] (SAD), [[delayed sleep phase syndrome]] (DSPS) and other [[circadian rhythm disorder]]s.<ref name=brynmawr>{{cite web | Sabah Quraishi | title =Circadian Rhythms and Sleep | publisher=Serendip | work = Circadian Rhythms and Sleep | url=http://serendip.brynmawr.edu/bb/neuro/neuro01/web1/Quirashi.html | year = 2007 | accessdate=2007-09-19}}</ref> Circadian rhythms also play a part in the [[reticular activating system]], which is crucial for maintaining a state of consciousness. In addition, a reversal in the sleep–wake cycle may be a sign or complication of [[uremia]],<ref>{{cite web |url= http://www.emedicine.com/emerg/topic500.htm |title= Renal Failure, Acute |accessdate= 2008-08-03 |last= Sinert |first= Richard |coauthors= Peter R Peacock, Jr |date= May 10, 2006 |work= |publisher= eMedicine from WebMD |doi= |quote= }}</ref> [[azotemia]] or [[acute renal failure]]. |
||
Studies have also shown that light has a direct effect on human health because of the way it influences the circadian rhythms. |
Studies have also shown that light has a direct effect on human health because of the way it influences the circadian rhythms.<ref>{{cite journal |author=Figueiro MG, Bierman A, Plitnick B, Rea MS |title=Preliminary evidence that both blue and red light can induce alertness at night |journal=BMC Neuroscience |volume=10 |issue= |pages=105 |year=2009 |pmid=19712442 |pmc=2744917 |doi=10.1186/1471-2202-10-105}}</ref><ref>{{cite journal |author=Figueiro MG, Rea MS, Bullough JD |title=Does architectural lighting contribute to breast cancer? |journal=Journal of Carcinogenesis |volume=5 |issue= |pages=20 |year=2006 |pmid=16901343 |pmc=1557490 |doi=10.1186/1477-3163-5-20}}</ref><ref>Sloane PD, Figueiro MG, Cohen L. 2008. Light Therapy for Sleep Disorders and Depression in Older Adults. Clinical Geriatrics, March 2008:2-8.</ref><ref>{{cite journal |author=Figueiro MG, Bierman A, Plitnick B, Rea MS |title=Preliminary evidence that both blue and red light can induce alertness at night |journal=BMC Neuroscience |volume=10 |issue= |pages=105 |year=2009 |pmid=19712442 |pmc=2744917 |doi=10.1186/1471-2202-10-105}}</ref> |
||
=== Disruption === |
=== Disruption === |
||
Disruption to rhythms usually has a negative effect. Many travellers have experienced the condition known as [[jet lag]], with its associated symptoms of [[fatigue (physical)|fatigue]], disorientation and [[insomnia]]. |
Disruption to rhythms usually has a negative effect. Many travellers have experienced the condition known as [[jet lag]], with its associated symptoms of [[fatigue (physical)|fatigue]], disorientation and [[insomnia]]. |
||
A number of other disorders, for example [[bipolar disorder]] and some [[sleep disorder]]s, are associated with irregular or pathological functioning of circadian rhythms. Recent research suggests that circadian rhythm disturbances found in [[bipolar disorder]] are positively influenced by [[lithium pharmacology|lithium]]'s effect on clock genes.<ref> |
A number of other disorders, for example [[bipolar disorder]] and some [[sleep disorder]]s, are associated with irregular or pathological functioning of circadian rhythms. Recent research suggests that circadian rhythm disturbances found in [[bipolar disorder]] are positively influenced by [[lithium pharmacology|lithium]]'s effect on clock genes.<ref>{{cite journal |author=Yin L, Wang J, Klein PS, Lazar MA |title=Nuclear receptor Rev-erbalpha is a critical lithium-sensitive component of the circadian clock |journal=Science |volume=311 |issue=5763 |pages=1002–5 |year=2006 |month=February |pmid=16484495 |doi=10.1126/science.1121613 |laysummary=http://www.nimh.nih.gov/science-news/2006/lithium-blocks-enzyme-to-help-cells-clocks-keep-on-tickin.shtml |laysource=[[National Institute of Mental Health]] |laydate=February 17, 2006}}</ref> |
||
Disruption to rhythms in the longer term is believed to have significant adverse health consequences on peripheral organs outside the brain, particularly in the development or exacerbation of cardiovascular disease |
Disruption to rhythms in the longer term is believed to have significant adverse health consequences on peripheral organs outside the brain, particularly in the development or exacerbation of cardiovascular disease.<ref>{{cite journal |author=Martino TA, Oudit GY, Herzenberg AM, ''et al.'' |title=Circadian rhythm disorganization produces profound cardiovascular and renal disease in hamsters |journal=American Journal of Physiology. Regulatory, Integrative and Comparative Physiology |volume=294 |issue=5 |pages=R1675–83 |year=2008 |month=May |pmid=18272659 |doi=10.1152/ajpregu.00829.2007}}</ref> The suppression of melatonin production associated with the disruption of the circadian rhythm may increase the risk of developing cancer.<ref>{{cite journal |author=Straif K, Baan R, Grosse Y, ''et al.'' |title=Carcinogenicity of shift-work, painting, and fire-fighting |journal=The Lancet Oncology |volume=8 |issue=12 |pages=1065–6 |year=2007 |month=December |pmid=19271347 |laysummary=http://www.webmd.com/cancer/news/20071130/night_shift-work-may-cause-cancer |laysource=[[WebMD]] |laydate=November 30, 2007}}</ref> |
||
Carcinogenicity of shift-work, painting, and fire-fighting. [http://www.iarc.fr/en/Media-Centre/IARC-Press-Releases/Recent-Releases/IARC-Monographs-Programme-finds-cancer-hazards-associated-with-shiftwork-painting-and-firefighting] Lancet Oncol. 2007; 12(8):1065-1066.</ref><ref>[http://www.webmd.com/cancer/news/20071130/night_shift-work-may-cause-cancer?src=RSS_PUBLIC WebMD: Night Shift Work May Cause Cancer]</ref> |
|||
=== Effect of drugs === |
=== Effect of drugs === |
||
| ⚫ | Circadian rhythms and clock genes expressed in brain regions outside the SCN may significantly influence the effects produced by drugs such as [[cocaine]].<ref>{{cite journal |author=Uz T, Akhisaroglu M, Ahmed R, Manev H |title=The pineal gland is critical for circadian Period1 expression in the striatum and for circadian cocaine sensitization in mice |journal=Neuropsychopharmacology |volume=28 |issue=12 |pages=2117–23 |year=2003 |month=December |pmid=12865893 |doi=10.1038/sj.npp.1300254}}</ref><ref>{{cite journal |author=Kurtuncu M, Arslan AD, Akhisaroglu M, Manev H, Uz T |title=Involvement of the pineal gland in diurnal cocaine reward in mice |journal=European Journal of Pharmacology |volume=489 |issue=3 |pages=203–5 |year=2004 |month=April |pmid=15087244 |doi=10.1016/j.ejphar.2004.03.010}}</ref> Moreover, genetic manipulations of clock genes profoundly affect cocaine's actions.<ref>{{cite journal |author=McClung CA, Sidiropoulou K, Vitaterna M, ''et al.'' |title=Regulation of dopaminergic transmission and cocaine reward by the Clock gene |journal=Proceedings of the National Academy of Sciences of the United States of America |volume=102 |issue=26 |pages=9377–81 |year=2005 |month=June |pmid=15967985 |pmc=1166621 |doi=10.1073/pnas.0503584102}}</ref> |
||
| ⚫ | Circadian rhythms and clock genes expressed in brain regions outside the SCN may significantly influence the effects produced by drugs such as [[cocaine]].<ref>{{cite journal |author=Uz T, Akhisaroglu M, Ahmed R, Manev H |title=The pineal gland is critical for circadian Period1 expression in the striatum and for circadian cocaine sensitization in mice |journal=Neuropsychopharmacology |volume=28 |issue=12 |pages=2117–23 |year=2003 |pmid=12865893 |doi=10.1038/sj.npp.1300254}}</ref><ref>{{cite journal |author=Kurtuncu M, Arslan |
||
== See also == |
== See also == |
||
* [[Actigraphy]] (also known as Actimetry) |
* [[Actigraphy]] (also known as Actimetry) |
||
* [[Advanced sleep phase syndrome]] |
* [[Advanced sleep phase syndrome]] |
||
| Line 180: | Line 149: | ||
{{refbegin|colwidth=40em}} |
{{refbegin|colwidth=40em}} |
||
* Aschoff J (ed.) (1965) ''Circadian Clocks''. North Holland Press, Amsterdam |
* Aschoff J (ed.) (1965) ''Circadian Clocks''. North Holland Press, Amsterdam |
||
* Avivi A, Albrecht U, Oster H, Joel A, Beiles A, Nevo E |
*{{cite journal |author=Avivi A, Albrecht U, Oster H, Joel A, Beiles A, Nevo E |title=Biological clock in total darkness: the Clock/MOP3 circadian system of the blind subterranean mole rat |journal=Proceedings of the National Academy of Sciences of the United States of America |volume=98 |issue=24 |pages=13751–6 |year=2001 |month=November |pmid=11707566 |pmc=61113 |doi=10.1073/pnas.181484498}} |
||
* Avivi A, Oster H, Joel A, Beiles A, Albrecht U, Nevo E |
*{{cite journal |author=Avivi A, Oster H, Joel A, Beiles A, Albrecht U, Nevo E |title=Circadian genes in a blind subterranean mammal II: conservation and uniqueness of the three Period homologs in the blind subterranean mole rat, Spalax ehrenbergi superspecies |journal=Proceedings of the National Academy of Sciences of the United States of America |volume=99 |issue=18 |pages=11718–23 |year=2002 |month=September |pmid=12193657 |pmc=129335 |doi=10.1073/pnas.182423299}} |
||
* Ditty JL, Williams SB, Golden SS |
*{{cite journal |author=Ditty JL, Williams SB, Golden SS |title=A cyanobacterial circadian timing mechanism |journal=Annual Review of Genetics |volume=37 |issue= |pages=513–43 |year=2003 |pmid=14616072 |doi=10.1146/annurev.genet.37.110801.142716}} |
||
* Dunlap JC, Loros J, DeCoursey PJ (2003) ''Chronobiology: Biological Timekeeping''. Sinauer, Sunderland |
* Dunlap JC, Loros J, DeCoursey PJ (2003) ''Chronobiology: Biological Timekeeping''. Sinauer, Sunderland |
||
* Dvornyk V, Vinogradova |
*{{cite journal |author=Dvornyk V, Vinogradova O, Nevo E |title=Origin and evolution of circadian clock genes in prokaryotes |journal=Proceedings of the National Academy of Sciences of the United States of America |volume=100 |issue=5 |pages=2495–500 |year=2003 |month=March |pmid=12604787 |pmc=151369 |doi=10.1073/pnas.0130099100}} |
||
* Koukkari WL, Sothern RB (2006) ''Introducing Biological Rhythms''. Springer, New York |
* Koukkari WL, Sothern RB (2006) ''Introducing Biological Rhythms''. Springer, New York |
||
* Martino T, Arab S, Straume M, |
*{{cite journal |author=Martino T, Arab S, Straume M, ''et al.'' |title=Day/night rhythms in gene expression of the normal murine heart |journal=Journal of Molecular Medicine |volume=82 |issue=4 |pages=256–64 |year=2004 |month=April |pmid=14985853 |doi=10.1007/s00109-003-0520-1}} |
||
* Refinetti R (2006) ''Circadian Physiology, 2nd ed''. CRC Press, Boca Raton |
* Refinetti R (2006) ''Circadian Physiology, 2nd ed''. CRC Press, Boca Raton |
||
* Takahashi JS, Zatz M |
*{{cite journal |author=Takahashi JS, Zatz M |title=Regulation of circadian rhythmicity |journal=Science |volume=217 |issue=4565 |pages=1104–11 |year=1982 |month=September |pmid=6287576 |doi=10.1126/science.6287576}} |
||
* Tomita J, Nakajima M, Kondo T, Iwasaki H |
*{{cite journal |author=Tomita J, Nakajima M, Kondo T, Iwasaki H |title=No transcription-translation feedback in circadian rhythm of KaiC phosphorylation |journal=Science |volume=307 |issue=5707 |pages=251–4 |year=2005 |month=January |pmid=15550625 |doi=10.1126/science.1102540}} |
||
* Moore-Ede |
*{{cite book |last1=Moore-Ede |first1=Martin C. |last2=Sulszman |first2=Frank M. |last3=Fuller |first3=Charles A. |year=1982 |title=The Clocks that Time Us: Physiology of the Circadian Timing System |publisher=Harvard University Press |location=Cambridge, MA |isbn=0-674-13581-4}} |
||
{{refend}} |
{{refend}} |
||
| Line 201: | Line 170: | ||
==External links== |
==External links== |
||
* {{dmoz|Health/Conditions_and_Diseases/Sleep_Disorders/Biological_Rhythms}} |
* {{dmoz|Health/Conditions_and_Diseases/Sleep_Disorders/Biological_Rhythms}} |
||
*{{cite journal |
*{{cite journal |author=Forger D, Gonze D, Virshup D, Welsh DK |title=Beyond intuitive modeling: combining biophysical models with innovative experiments to move the circadian clock field forward |journal=Journal of Biological Rhythms |volume=22 |issue=3 |pages=200–10 |year=2007 |month=June |pmid=17517910 |doi=10.1177/0748730407301823}} |
||
*{{cite journal | author=Rodrigo G, Carrera J, Jaramillo A |title=Evolutionary mechanisms of circadian clocks |journal= |
*{{cite journal | author=Rodrigo G, Carrera J, Jaramillo A |title=Evolutionary mechanisms of circadian clocks |journal=Central European Journal of Biology |volume=2 |issue= 2 |pages= 233–253 |year= 2007 |doi=10.2478/s11535-007-0016-z }} |
||
{{DEFAULTSORT:Circadian Rhythm}} |
{{DEFAULTSORT:Circadian Rhythm}} |
||
Revision as of 09:05, 19 February 2010

A circadian rhythm is a roughly 24-hour cycle in the biochemical, physiological or behavioural processes of living entities, including plants, animals, fungi and cyanobacteria (see bacterial circadian rhythms). The term "circadian", coined by Franz Halberg,[1] comes from the Latin circa, "around", and diem or dies, "day", meaning literally "approximately one day". The formal study of biological temporal rhythms such as daily, tidal, weekly, seasonal, and annual rhythms, is called chronobiology.
Although circadian rhythms are endogenous, they are adjusted (entrained) to the environment by external cues called zeitgebers, the primary one of which is daylight.
History
The earliest known account of a circadian rhythm dates from the 4th century BC, when Androsthenes, a ship captain serving under Alexander the Great, described diurnal leaf movements of the tamarind tree.[2] The first modern observation of endogenous circadian oscillation was by the French scientist Jean-Jacques d'Ortous de Mairan in the 1700s; he noted that 24-hour patterns in the movement of the leaves of the plant Mimosa pudica continued even when the plants were isolated from external stimuli.
In 1918, J. S. Szymanski showed that animals are capable of maintaining 24-hour activity patterns in the absence of external cues such as light and changes in temperature.[3] Joseph Takahashi discovered the genetic basis for the rodent circadian rhythm in 1994.[4][5]
Criteria
To differentiate genuinely endogenous circadian rhythms from coincidental or apparent ones, three general criteria must be met: 1) the rhythms persist in the absence of cues, 2) they persist equally precisely over a range of temperatures, and 3) the rhythms can be adjusted to match the local time:
- The rhythm persists in constant conditions (for example, constant dark) with a period of about 24 hours. The rationale for this criterion is to distinguish circadian rhythms from those "apparent" rhythms that are merely responses to external periodic cues. A rhythm cannot be declared to be endogenous unless it has been tested in conditions without external periodic input.
- The rhythm is temperature-compensated, i.e., it maintains the same period over a range of temperatures. The rationale for this criterion is to distinguish circadian rhythms from other biological rhythms arising due to the circular nature of a reaction pathway. At a low enough or high enough temperature, the period of a circular reaction may reach 24 hours, but it will be merely coincidental.
- The rhythm can be reset by exposure to an external stimulus. The rationale for this criterion is to distinguish circadian rhythms from other imaginable endogenous 24-hour rhythms that are immune to resetting by external cues and, hence, do not serve the purpose of estimating the local time. Travel across time zones illustrates the necessity of the ability to adjust the biological clock so that it can reflect the local time and anticipate what will happen next. Until rhythms are reset, a person usually experiences jet lag.
Origin
This section needs additional citations for verification. (October 2007) |
Photosensitive proteins and circadian rhythms are believed to have originated in the earliest cells, with the purpose of protecting the replicating of DNA from high ultraviolet radiation during the daytime. As a result, replication was relegated to the dark. The fungus Neurospora, which exists today, retains this clock-regulated mechanism.
Circadian rhythms allow organisms to anticipate and prepare for precise and regular environmental changes; they have great value in relation to the outside world. The rhythmicity appears to be as important in regulating and coordinating internal metabolic processes, as in coordinating with the environment.[6] This is suggested by the maintenance (heritability) of circadian rhythms in fruit flies after several hundred generations in constant laboratory conditions,[7] as well as in creatures in constant darkness in the wild, and by the experimental elimination of behavioural but not physiological circadian rhythms in quail.[8][9]
The simplest known circadian clock is that of the prokaryotic cyanobacteria. Recent research has demonstrated that the circadian clock of Synechococcus elongatus can be reconstituted in vitro with just the three proteins of their central oscillator. This clock has been shown to sustain a 22-hour rhythm over several days upon the addition of ATP. Previous explanations of the prokaryotic circadian timekeeper were dependent upon a DNA transcription / translation feedback mechanism.
It is an unanswered question whether circadian clocks in eukaryotic organisms require translation/transcription-derived oscillations, for, although the circadian systems of eukaryotes and prokaryotes have the same basic architecture (input – central oscillator – output), they do not share any homology. This implies probable independent origins.
In 1971, Ronald J. Konopka and Seymour Benzer first identified a genetic component of the biological clock using the fruit fly as a model system. Three mutant lines of flies displayed aberrant behaviour: one had a shorter period, another had a longer one, and the third had none. All three mutations mapped to the same gene, which was named period.[10] The same gene was identified to be defective in the sleep disorder FASPS (Familial advanced sleep phase syndrome) in human beings thirty years later, underscoring the conserved nature of the molecular circadian clock through evolution. Many more genetic components of the biological clock are now known. Their interactions result in an interlocked feedback loop of gene products resulting in periodic fluctuations that the cells of the body interpret as a specific time of the day.
A great deal of research on biological clocks was done in the latter half of the 20th century. It is now known that the molecular circadian clock can function within a single cell; i.e., it is cell-autonomous.[11] At the same time, different cells may communicate with each other resulting in a synchronized output of electrical signaling. These may interface with endocrine glands of the brain to result in periodic release of hormones. The receptors for these hormones may be located far across the body and synchronize the peripheral clocks of various organs. Thus, the information of the time of the day as relayed by the eyes travels to the clock in the brain, and, through that, clocks in the rest of the body may be synchronized. This is how the timing of, for example, sleep/wake, body temperature, thirst, and appetite are coordinately controlled by the biological clock.
Importance in animals
Circadian rhythmicity is present in the sleeping and feeding patterns of animals, including human beings. There are also clear patterns of core body temperature, brain wave activity, hormone production, cell regeneration and other biological activities. In addition, photoperiodism, the physiological reaction of organisms to the length of day or night, is vital to both plants and animals, and the circadian system plays a role in the measurement and interpretation of day length.
Timely prediction of seasonal periods of weather conditions, food availability or predator activity is crucial for survival of many species. Although not the only parameter, the changing length of the photoperiod ('daylength') is the most predictive environmental cue for the seasonal timing of physiology and behavior, most notably for timing of migration, hibernation and reproduction.[12]
Impact of light–dark cycle
The rhythm is linked to the light–dark cycle. Animals, including humans, kept in total darkness for extended periods eventually function with a freerunning rhythm. Each "day", their sleep cycle is pushed back or forward, depending on whether their endogenous period is shorter or longer than 24 hours. The environmental cues that each day reset the rhythms are called Zeitgebers (from the German, Time Givers).[13] It is interesting to note that totally-blind subterranean mammals (e.g., blind mole rat Spalax sp.) are able to maintain their endogenous clocks in the apparent absence of external stimuli. Although they lack image-forming eyes, their photoreceptors (detect light) are still functional; as well, they do surface periodically.[citation needed]
Freerunning organisms that normally have one consolidated sleep episode will still have it when in an environment shielded from external cues, but the rhythm is, of course, not entrained to the 24-hour light/dark cycle in nature. The sleep–wake rhythm may, in these circumstances, become out of phase with other circadian or ultradian rhythms such as temperature and digestion.[citation needed]
Recent research has influenced the design of spacecraft environments, as systems that mimic the light/dark cycle have been found to be highly beneficial to astronauts.[citation needed]
Arctic animals
Norwegian researchers at the University of Tromsø have shown that some Arctic animals (ptarmigan, reindeer) show circadian rhythms only in the parts of the year that have daily sunrises and sunsets. In one study of reindeer, animals at 70 degrees North showed circadian rhythms in the autumn, winter, and spring, but not in the summer. Reindeer at 78 degrees North showed such rhythms only autumn and spring. The researchers suspect that other Arctic animals as well may not show circadian rhythms in the constant light of summer and the constant dark of winter.[14][15]
However, another study in northern Alaska found that ground squirrels and porcupines strictly maintained their circadian rhythms through 82 days and nights of sunshine. The researchers speculate that these two small mammals see that the apparent distance between the sun and the horizon is shortest once a day, and, thus, a sufficient signal to adjust by.[16]
Butterfly migration
The navigation of the fall migration of the Eastern North American monarch butterfly (Danaus plexippus) to their overwintering grounds in central Mexico uses a time-compensated sun compass that depends upon a circadian clock in their antennae.[17][18]
Biological clock in mammals
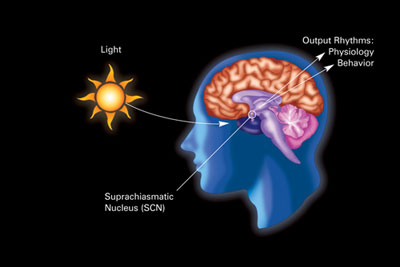
The primary circadian "clock" in mammals is located in the suprachiasmatic nucleus (or nuclei) (SCN), a pair of distinct groups of cells located in the hypothalamus. Destruction of the SCN results in the complete absence of a regular sleep–wake rhythm. The SCN receives information about illumination through the eyes. The retina of the eye contains not only the "classical" photoreceptors which are used for vision but ganglion cells which respond to light and are called photosensitive ganglion cells.
These cells contain the photo pigment melanopsin and their signals follow a pathway called the retinohypothalamic tract, leading to the SCN. If cells from the SCN are removed and cultured, they maintain their own rhythm in the absence of external cues.
The SCN takes the information on the lengths of the day and night from the retina, interprets it, and passes it on to the pineal gland, a tiny structure shaped like a pine cone and located on the epithalamus. In response the pineal secretes the hormone melatonin. Secretion of melatonin peaks at night and ebbs during the day and its presence provides information about night-length.
The circadian rhythms of humans can be entrained to slightly shorter and longer periods than the Earth's 24 hours. Researchers at Harvard have recently shown that human subjects can at least be entrained to a 23.5-hour cycle and a 24.65-hour cycle (the latter being the natural solar day-night cycle on the planet Mars).[19]
Determining the human circadian rhythm
The classic phase markers for measuring the timing of a mammal's circadian rhythm are[20]
- melatonin secretion by the pineal gland and
- core body temperature.
For temperature studies, people must remain awake but calm and semi-reclined in near darkness while their rectal temperatures are taken continuously. The average human adult's temperature reaches its minimum at about 05:00 (5 a.m.), about two hours before habitual wake time, though variation is great among normal chronotypes.
Melatonin is absent from the system or undetectably low during daytime. Its onset in dim light, dim-light melatonin onset (DLMO), at about 21:00 (9 p.m.) can be measured in the blood or the saliva. Its major metabolite can also be measured in morning urine. Both DLMO and the midpoint (in time) of the presence of the hormone in the blood or saliva have been used as circadian markers.
However, newer research indicates that the melatonin offset may be the most reliable marker. Benloucif et al. in Chicago in 2005 found that melatonin phase markers were more stable and more highly correlated with the timing of sleep than the core temperature minimum. They found that both sleep offset and melatonin offset were more strongly correlated with the various phase markers than sleep onset. In addition, the declining phase of the melatonin levels was more reliable and stable than the termination of melatonin synthesis.[20]
One method used for measuring melatonin offset is to analyse a sequence of urine samples throughout the morning for the presence of the melatonin metabolite 6-sulphatoxymelatonin (aMT6s). Laberge et al. in Quebec in 1997 used this method in a study that confirmed the frequently found delayed circadian phase in healthy adolescents.[21]
Outside the "master clock"
More-or-less independent circadian rhythms are found in many organs and cells in the body outside the suprachiasmatic nuclei (SCN), the "master clock". These clocks, called peripheral oscillators, are found in the oesophagus, lungs, liver, pancreas, spleen, thymus, and the skin.[22] Though oscillators in the skin respond to light, a systemic influence has not been proven so far.[23][24] There is some evidence that also the olfactory bulb and prostate may experience oscillations when cultured, suggesting that also these structures may be weak oscillators.
Furthermore, liver cells, for example, appear to respond to feeding rather than to light. Cells from many parts of the body appear to have freerunning rhythms.
Light and the biological clock
Light resets the biological clock in accordance with the phase response curve (PRC). Depending on the timing, light can advance or delay the circadian rhythm. Both the PRC and the required illuminance vary from species to species and lower light levels are required to reset the clocks in nocturnal rodents than in humans.
Lighting levels that affect circadian rhythm in humans are higher than the levels usually used in artificial lighting in homes. According to some researchers[25] the illumination intensity that excites the circadian system has to reach up to 1000 lux striking the retina. In addition to light intensity, wavelength (or colour) of light is a factor in the entrainment of the body clock. Melanopsin is most efficiently excited by blue light, 420–440 nm[26] according to some researchers while others have reported 470–485 nm.
It is thought that the direction of the light may have an effect on entraining the circadian rhythm;[25] light coming from above, resembling an image of a bright sky, has greater effect than light entering our eyes from below.
According to a 2010 study completed by the Lighting Research Center, daylight has a direct effect on circadian rhythms and, consequently, on performance and well-being. The research showed that students who experience disruption in lighting schemes in the morning consequently experience disruption in sleeping patterns. The change in sleeping patterns may lead to negatively impacted student performance and alertness. Removing circadian light in the morning delays the dim light melatonin onset by 6 minutes a day, for a total of 30 minutes for five days.[27]
Enforced longer cycles
Modern research under very controlled conditions has shown the human period for adults to be just slightly longer than 24 hours on average. Czeisler et al. at Harvard found the range for normal, healthy adults of all ages to be quite narrow: 24 hours and 11 minutes ± 16 minutes. The "clock" resets itself daily to the 24-hour cycle of the Earth's rotation.[28]
The 28-hour day is presented as a concept of time management.[29] It builds on the fact that the week of seven days at 24 hours and a "week" of six days at 28 hours both equal a week of 168 hours. To live on the 28-hour day and six-day week would require staying awake for 19 to 20 hours and sleeping for eight to nine hours. Each "day" on this system has a unique light/dark pattern.
Studies by Nathaniel Kleitman[30] in 1938 and by Derk-Jan Dijk and Charles Czeisler[31][32] in 1994/5 have put human subjects on enforced 28-hour sleep–wake cycles, in constant dim light and with other time cues suppressed, for over a month. Because normal people cannot entrain to a 28-hour day,[33] this is referred to as a forced desynchrony protocol. Sleep and wake episodes are uncoupled from the endogenous circadian period of about 24.18 hours and researchers are allowed to assess the effects of circadian phase on aspects of sleep and wakefulness including sleep latency and other functions.[34]
Early research into circadian rhythms suggested that most people preferred a day closer to 25 hours when isolated from external stimuli like daylight and timekeeping. Early investigators determined the human circadian period to be 25 hours or more. They went to great lengths to shield subjects from time cues and daylight, but they were not aware of the effects of indoor electric lights. The subjects were allowed to turn on light when they were awake and to turn it off when they wanted to sleep. Electric light in the evening delayed their circadian phase. These results became well known.[28] Researchers allowed subjects to keep electric lighting on in the evening, as it was thought at that time that a couple of 60W bulbs would not have a resetting effect on the circadian rhythms of humans. More recent research[citation needed] has shown that adults have a built-in day, which averages just over 24 hours, that indoor lighting does affect circadian rhythms and that most people attain their best-quality sleep during their chronotype-determined sleep periods.
Human health
Timing of medical treatment in coordination with the body clock may significantly increase efficacy and reduce drug toxicity or adverse reactions. For example, appropriately timed treatment with angiotensin converting enzyme inhibitors (ACEi) may reduce nocturnal blood pressure and also benefit left ventricular (reverse) remodelling. [citation needed]

A number of studies have concluded that a short period of sleep during the day, a power-nap, does not have any effect on normal circadian rhythm, but can decrease stress and improve productivity.[35][36]
There are many health problems associated with disturbances of the human circadian rhythm, such as seasonal affective disorder (SAD), delayed sleep phase syndrome (DSPS) and other circadian rhythm disorders.[37] Circadian rhythms also play a part in the reticular activating system, which is crucial for maintaining a state of consciousness. In addition, a reversal in the sleep–wake cycle may be a sign or complication of uremia,[38] azotemia or acute renal failure.
Studies have also shown that light has a direct effect on human health because of the way it influences the circadian rhythms.[39][40][41][42]
Disruption
Disruption to rhythms usually has a negative effect. Many travellers have experienced the condition known as jet lag, with its associated symptoms of fatigue, disorientation and insomnia.
A number of other disorders, for example bipolar disorder and some sleep disorders, are associated with irregular or pathological functioning of circadian rhythms. Recent research suggests that circadian rhythm disturbances found in bipolar disorder are positively influenced by lithium's effect on clock genes.[43]
Disruption to rhythms in the longer term is believed to have significant adverse health consequences on peripheral organs outside the brain, particularly in the development or exacerbation of cardiovascular disease.[44] The suppression of melatonin production associated with the disruption of the circadian rhythm may increase the risk of developing cancer.[45]
Effect of drugs
Circadian rhythms and clock genes expressed in brain regions outside the SCN may significantly influence the effects produced by drugs such as cocaine.[46][47] Moreover, genetic manipulations of clock genes profoundly affect cocaine's actions.[48]
See also
- Actigraphy (also known as Actimetry)
- Advanced sleep phase syndrome
- ARNTL
- ARNTL2
- Bacterial circadian rhythms
- Chronobiology
- Chronotype
- Circadian oscillator
- Circadian rhythm sleep disorders
- Cryptochrome
- CRY1 and CRY2, the cryptochrome family genes
- Delayed sleep phase syndrome
- Diurnal cycle
- Jet lag
- Light effects on circadian rhythm
- Impact of Lighting and Daylight in K-12 School Buildings
- Lighting Research Center, Light & Health Program: http://www.lrc.rpi.edu/programs/lightHealth/index.asp
- PER1, PER2, and PER3, the period family genes
- Power-nap
References
Bibliography
- Aschoff J (ed.) (1965) Circadian Clocks. North Holland Press, Amsterdam
- Avivi A, Albrecht U, Oster H, Joel A, Beiles A, Nevo E (2001). "Biological clock in total darkness: the Clock/MOP3 circadian system of the blind subterranean mole rat". Proceedings of the National Academy of Sciences of the United States of America. 98 (24): 13751–6. doi:10.1073/pnas.181484498. PMC 61113. PMID 11707566.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - Avivi A, Oster H, Joel A, Beiles A, Albrecht U, Nevo E (2002). "Circadian genes in a blind subterranean mammal II: conservation and uniqueness of the three Period homologs in the blind subterranean mole rat, Spalax ehrenbergi superspecies". Proceedings of the National Academy of Sciences of the United States of America. 99 (18): 11718–23. doi:10.1073/pnas.182423299. PMC 129335. PMID 12193657.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - Ditty JL, Williams SB, Golden SS (2003). "A cyanobacterial circadian timing mechanism". Annual Review of Genetics. 37: 513–43. doi:10.1146/annurev.genet.37.110801.142716. PMID 14616072.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Dunlap JC, Loros J, DeCoursey PJ (2003) Chronobiology: Biological Timekeeping. Sinauer, Sunderland
- Dvornyk V, Vinogradova O, Nevo E (2003). "Origin and evolution of circadian clock genes in prokaryotes". Proceedings of the National Academy of Sciences of the United States of America. 100 (5): 2495–500. doi:10.1073/pnas.0130099100. PMC 151369. PMID 12604787.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - Koukkari WL, Sothern RB (2006) Introducing Biological Rhythms. Springer, New York
- Martino T, Arab S, Straume M; et al. (2004). "Day/night rhythms in gene expression of the normal murine heart". Journal of Molecular Medicine. 82 (4): 256–64. doi:10.1007/s00109-003-0520-1. PMID 14985853.
{{cite journal}}: Explicit use of et al. in:|author=(help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - Refinetti R (2006) Circadian Physiology, 2nd ed. CRC Press, Boca Raton
- Takahashi JS, Zatz M (1982). "Regulation of circadian rhythmicity". Science. 217 (4565): 1104–11. doi:10.1126/science.6287576. PMID 6287576.
{{cite journal}}: Unknown parameter|month=ignored (help) - Tomita J, Nakajima M, Kondo T, Iwasaki H (2005). "No transcription-translation feedback in circadian rhythm of KaiC phosphorylation". Science. 307 (5707): 251–4. doi:10.1126/science.1102540. PMID 15550625.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - Moore-Ede, Martin C.; Sulszman, Frank M.; Fuller, Charles A. (1982). The Clocks that Time Us: Physiology of the Circadian Timing System. Cambridge, MA: Harvard University Press. ISBN 0-674-13581-4.
Notes
- ^ http://www.msi.umn.edu/~halberg Halberg Chronobiology Centre
- ^ Bretzl H. (1903). Botaniche Forchungen des Alexanderzuges. Leipzig: Teubner.[page needed]
- ^ Danchin, Antoine. "Important dates 1900-1919". HKU-Pasteur Research Centre. Paris. Retrieved 2008-01-12.
{{cite journal}}: Cite has empty unknown parameter:|month=(help) - ^ "Gene Discovered in Mice that Regulates Biological Clock". Chicago Tribune. April 29, 1994.
- ^ Vitaterna MH, King DP, Chang AM; et al. (1994). "Mutagenesis and mapping of a mouse gene, Clock, essential for circadian behavior". Science. 264 (5159): 719–25. doi:10.1126/science.8171325. PMID 8171325.
{{cite journal}}: Explicit use of et al. in:|author=(help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Sharma VK (2003). "Adaptive significance of circadian clocks". Chronobiology International. 20 (6): 901–19. doi:10.1081/CBI-120026099. PMID 14680135.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ (Sheeba et al. 1999)
- ^ Guyomarc'h et al. 1998[verification needed]
- ^ Zivkovic et al. 1999[verification needed]
- ^ Purves, Dale; et al. (2001) [2001]. "Molecular Mechanisms of Biological Clocks". NEUROSCIENCE (e-book) (second ed.). Sunderland, MA, U.S.A.: Sinauer Associates. ISBN 0-87893-742-0. Retrieved 2008-05-30.
{{cite book}}: Cite has empty unknown parameters:|month=and|origdate=(help); Explicit use of et al. in:|first=(help); External link in|chapterurl=|chapterurl=ignored (|chapter-url=suggested) (help) - ^ Nagoshi E, Saini C, Bauer C, Laroche T, Naef F, Schibler U (2004). "Circadian gene expression in individual fibroblasts: cell-autonomous and self-sustained oscillators pass time to daughter cells". Cell. 119 (5): 693–705. doi:10.1016/j.cell.2004.11.015. PMID 15550250.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Zivkovic, Bora "Coturnix" (2005-08-13 / July 25, 2007). "Clock Tutorial #16: Photoperiodism - Models and Experimental Approaches". A Blog Around the Clock. ScienceBlogs. Retrieved 2007-12-09.
{{cite web}}: Check date values in:|date=(help) - ^ "Circadian rhythms". Rapid eye movement (REM) sleep. Armenian Medical Network. 2007. Retrieved 2007-09-19.
{{cite web}}: Text "Shneerson JM, Ohayon MM, Carskadon MA" ignored (help) - ^ Spilde, Ingrid (December 2005). "Reinsdyr uten døgnrytme" (in Language: Norwegian and Bokmål). forskning.no. Retrieved 2007-11-24.
{{cite news}}: CS1 maint: unrecognized language (link) - ^ Zivkovic, Bora, aka Coturnix, chronobiologist. "Circadian Rhythms, or Not, in Arctic Reindeer". A Blog around the Clock. ScienceBlogs.com. Retrieved 2007-11-24.
{{cite web}}: Cite has empty unknown parameter:|month=(help)CS1 maint: multiple names: authors list (link) - ^ Zivkovic, Bora, aka Coturnix, chronobiologist (2007-02-11). "Small Arctic Mammals Entrain to Something during the Long Summer Day". A Blog Around the Clock. ScienceBlogs.com. Retrieved 2007-11-26.
{{cite web}}: CS1 maint: multiple names: authors list (link) - ^ Merlin C, Gegear RJ, Reppert SM (2009). "Antennal circadian clocks coordinate sun compass orientation in migratory monarch butterflies". Science. 325 (5948): 1700–4. doi:10.1126/science.1176221. PMID 19779201.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Kyriacou CP (2009). "Physiology. Unraveling traveling". Science. 325 (5948): 1629–30. doi:10.1126/science.1178935. PMID 19779177.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Scheer FA, Wright KP, Kronauer RE, Czeisler CA (2007). "Plasticity of the intrinsic period of the human circadian timing system". PLos ONE. 2 (1): e721. doi:10.1371/journal.pone.0000721. PMC 1934931. PMID 17684566.
{{cite journal}}: CS1 maint: multiple names: authors list (link) CS1 maint: unflagged free DOI (link) - ^ a b Benloucif S, Guico MJ, Reid KJ, Wolfe LF, L'hermite-Balériaux M, Zee PC (2005). "Stability of melatonin and temperature as circadian phase markers and their relation to sleep times in humans". Journal of Biological Rhythms. 20 (2): 178–88. doi:10.1177/0748730404273983. PMID 15834114.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Laberge, L. (1997). "Phase delay of 6-sulphatoxymelatonin in normal adolescents". Sleep Research. 26. Québec, Canada: Centre d'etude du Sommeil, Hopital du Sacre-Coeur,
Département de Psychologie, Département de Pharmacologie, Departement de Psychiatrie, Université de Montréal: 727. Retrieved 2007-12-18.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help); line feed character in|publisher=at position 51 (help) - ^ Zanello SB, Jackson DM, Holick MF (2000). "Expression of the circadian clock genes clock and period1 in human skin". The Journal of Investigative Dermatology. 115 (4): 757–60. doi:10.1046/j.1523-1747.2000.00121.x. PMID 10998156.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Kawara S, Mydlarski R, Mamelak AJ; et al. (2002). "Low-dose ultraviolet B rays alter the mRNA expression of the circadian clock genes in cultured human keratinocytes". The Journal of Investigative Dermatology. 119 (6): 1220–3. doi:10.1046/j.1523-1747.2002.19619.x. PMID 12485420.
{{cite journal}}: Explicit use of et al. in:|author=(help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Campbell SS, Murphy PJ (1998). "Extraocular circadian phototransduction in humans". Science. 279 (5349): 396–9. doi:10.1126/science.279.5349.396. PMID 9430592.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ a b Semjonova, Milena (2003). "Healthy Lighting, from a lighting designer's perspective" (PDF). Milena Lighting Design.
- ^ Newman LA, Walker MT, Brown RL, Cronin TW, Robinson PR (2003). "Melanopsin forms a functional short-wavelength photopigment". Biochemistry. 42 (44): 12734–8. doi:10.1021/bi035418z. PMID 14596587.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Figueiro MG, Rea MS (2010). "Lack of short-wavelength light during the school day delays dim light melatonin onset (DLMO) in middle school students". Neuro Endocrinology Letters. 31 (1): 4. PMID 20150866.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ a b "Human Biological Clock Set Back an Hour". 1999. Retrieved 2007-09-23.
The variation between our subjects, with a 95 percent level of confidence, was no more than plus or minus 16 minutes, a remarkably small range.
{{cite web}}: Text "Charles A. Czeisler MD, PhD" ignored (help) - ^ Digital Beat Productions (1997). "28 Hour Day". Retrieved 2008-02-19.
- ^ Kleitman, Nathaniel (1962). Sleep and Wakefullness ed 2. Chicago: University of Chicago Press.
- ^ Dijk DJ, Czeisler CA (1994). "Paradoxical timing of the circadian rhythm of sleep propensity serves to consolidate sleep and wakefulness in humans". Neuroscience Letters. 166 (1): 63–8. doi:10.1016/0304-3940(94)90841-9. PMID 8190360.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Dijk DJ, Czeisler CA (1995). "Contribution of the circadian pacemaker and the sleep homeostat to sleep propensity, sleep structure, electroencephalographic slow waves, and sleep spindle activity in humans". The Journal of Neuroscience. 15 (5 Pt 1): 3526–38. PMID 7751928.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Cromie, William J. (1999-07-15). "Human Biological Clock Set Back an Hour". The Harvard University Gazette. Retrieved 2008-02-19.
- ^ Aldrich, Michael S (1999). Sleep medicine. New York: Oxford University Press. ISBN 0-19-512957-1.
- ^ Pilcher JJ, Michalowski KR, Carrigan RD (2001). "The prevalence of daytime napping and its relationship to nighttime sleep". Behavioral Medicine. 27 (2): 71–6. PMID 11763827.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ "Power-Napping: Effects on Cognitive Ability and Stress Levels Among College Students". Power-Napping: Effects on Cognitive Ability and Stress Levels Among College Students. Liberty University. 2007. Retrieved 2008-11-11.
{{cite web}}: Text "Emily Rolston, Judy R. Sandlin, Michael Sandlin, and Rosanne Keathley" ignored (help) - ^ "Circadian Rhythms and Sleep". Circadian Rhythms and Sleep. Serendip. 2007. Retrieved 2007-09-19.
{{cite web}}: Text "Sabah Quraishi" ignored (help) - ^ Sinert, Richard (May 10, 2006). "Renal Failure, Acute". eMedicine from WebMD. Retrieved 2008-08-03.
{{cite web}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Figueiro MG, Bierman A, Plitnick B, Rea MS (2009). "Preliminary evidence that both blue and red light can induce alertness at night". BMC Neuroscience. 10: 105. doi:10.1186/1471-2202-10-105. PMC 2744917. PMID 19712442.
{{cite journal}}: CS1 maint: multiple names: authors list (link) CS1 maint: unflagged free DOI (link) - ^ Figueiro MG, Rea MS, Bullough JD (2006). "Does architectural lighting contribute to breast cancer?". Journal of Carcinogenesis. 5: 20. doi:10.1186/1477-3163-5-20. PMC 1557490. PMID 16901343.
{{cite journal}}: CS1 maint: multiple names: authors list (link) CS1 maint: unflagged free DOI (link) - ^ Sloane PD, Figueiro MG, Cohen L. 2008. Light Therapy for Sleep Disorders and Depression in Older Adults. Clinical Geriatrics, March 2008:2-8.
- ^ Figueiro MG, Bierman A, Plitnick B, Rea MS (2009). "Preliminary evidence that both blue and red light can induce alertness at night". BMC Neuroscience. 10: 105. doi:10.1186/1471-2202-10-105. PMC 2744917. PMID 19712442.
{{cite journal}}: CS1 maint: multiple names: authors list (link) CS1 maint: unflagged free DOI (link) - ^ Yin L, Wang J, Klein PS, Lazar MA (2006). "Nuclear receptor Rev-erbalpha is a critical lithium-sensitive component of the circadian clock". Science. 311 (5763): 1002–5. doi:10.1126/science.1121613. PMID 16484495.
{{cite journal}}: Unknown parameter|laydate=ignored (help); Unknown parameter|laysource=ignored (help); Unknown parameter|laysummary=ignored (help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Martino TA, Oudit GY, Herzenberg AM; et al. (2008). "Circadian rhythm disorganization produces profound cardiovascular and renal disease in hamsters". American Journal of Physiology. Regulatory, Integrative and Comparative Physiology. 294 (5): R1675–83. doi:10.1152/ajpregu.00829.2007. PMID 18272659.
{{cite journal}}: Explicit use of et al. in:|author=(help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Straif K, Baan R, Grosse Y; et al. (2007). "Carcinogenicity of shift-work, painting, and fire-fighting". The Lancet Oncology. 8 (12): 1065–6. PMID 19271347.
{{cite journal}}: Explicit use of et al. in:|author=(help); Unknown parameter|laydate=ignored (help); Unknown parameter|laysource=ignored (help); Unknown parameter|laysummary=ignored (help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Uz T, Akhisaroglu M, Ahmed R, Manev H (2003). "The pineal gland is critical for circadian Period1 expression in the striatum and for circadian cocaine sensitization in mice". Neuropsychopharmacology. 28 (12): 2117–23. doi:10.1038/sj.npp.1300254. PMID 12865893.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Kurtuncu M, Arslan AD, Akhisaroglu M, Manev H, Uz T (2004). "Involvement of the pineal gland in diurnal cocaine reward in mice". European Journal of Pharmacology. 489 (3): 203–5. doi:10.1016/j.ejphar.2004.03.010. PMID 15087244.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ McClung CA, Sidiropoulou K, Vitaterna M; et al. (2005). "Regulation of dopaminergic transmission and cocaine reward by the Clock gene". Proceedings of the National Academy of Sciences of the United States of America. 102 (26): 9377–81. doi:10.1073/pnas.0503584102. PMC 1166621. PMID 15967985.
{{cite journal}}: Explicit use of et al. in:|author=(help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link)
External links
- Circadian rhythm at Curlie
- Forger D, Gonze D, Virshup D, Welsh DK (2007). "Beyond intuitive modeling: combining biophysical models with innovative experiments to move the circadian clock field forward". Journal of Biological Rhythms. 22 (3): 200–10. doi:10.1177/0748730407301823. PMID 17517910.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - Rodrigo G, Carrera J, Jaramillo A (2007). "Evolutionary mechanisms of circadian clocks". Central European Journal of Biology. 2 (2): 233–253. doi:10.2478/s11535-007-0016-z.
{{cite journal}}: CS1 maint: multiple names: authors list (link)
