Melanopsin
Melanopsin is a type of photopigment belonging to a larger family of light-sensitive retinal proteins called opsins and encoded by the gene Opn4.[5] In the mammalian retina, there are two additional categories of opsins, both involved in the formation of visual images: rhodopsin and photopsin (types I, II, and III) in the rod and cone photoreceptor cells, respectively.
In humans, melanopsin is found in intrinsically photosensitive retinal ganglion cells (ipRGCs).[6] It is also found in the iris of mice and primates.[7] Melanopsin is also found in rats, amphioxus, and other chordates.[8] ipRGCs are photoreceptor cells which are particularly sensitive to the absorption of short-wavelength (blue) visible light and communicate information directly to the area of the brain called the suprachiasmatic nucleus (SCN), also known as the central "body clock", in mammals.[9] Melanopsin plays an important non-image-forming role in the setting of circadian rhythms as well as other functions. Mutations in the Opn4 gene can lead to clinical disorders, such as Seasonal Affective Disorder (SAD).[10] According to one study, melanopsin has been found in eighteen sites in the human brain (outside the retinohypothalamic tract), intracellularly, in a granular pattern, in the cerebral cortex, the cerebellar cortex and several phylogenetically old regions, primarily in neuronal soma, not in nuclei.[11] Melanopsin is also expressed in human cones. However, only 0.11% to 0.55% of human cones express melanopsin and are exclusively found in the peripheral regions of the retina.[12] The human peripheral retina senses light at high intensities that is best explained by four different photopigment classes.[13]
Discovery
[edit]
Melanopsin was discovered by Ignacio Provencio as a new opsin in the melanophores, or light-sensitive skin cells, of the African clawed frog in 1998.[14] A year later, researchers found that mice without any rods or cones, the cells involved in image-forming vision, still entrained to a light-dark cycle.[15] This observation led to the conclusion that neither rods nor cones, located in the outer retina, are necessary for circadian entrainment and that a third class of photoreceptor exists in the mammalian eye.[5] Provencio and colleagues then found in 2000 that melanopsin is also present in mouse retina, specifically in ganglion cells, and that it mediates non-visual photoreceptive tasks.[16] Melanopsin is encoded by the Opn4 gene with orthologs in a variety of organisms.[5]
These retinal ganglion cells were found to be innately photosensitive, since they responded to light even while isolated, and were thus named intrinsically photosensitive Retinal Ganglion Cells (ipRGCs).[17] They constitute a third class of photoreceptor cells in the mammalian retina, besides the already known rods and cones, and were shown to be the principal conduit for light input to circadian photoentrainment.[16] In fact, it was later demonstrated by Satchidananda Panda and colleagues that melanopsin pigment may be involved in entrainment of a circadian oscillator to light cycles in mammals since melanopsin was necessary for blind mice to respond to light.[18]
Species distribution
[edit]Mammals have orthologous melanopsin genes named Opn4m, which are derived from one branch of the Opn4 family, and are approximately 50-55% conserved.[19] However, non-mammalian vertebrates, including chickens and zebrafish, have another version of the melanopsin gene, Opn4x, which appears to have a distinct lineage that diverged from Opn4m about 360 million years ago.[20] Mammals lost the gene Opn4x relatively early in their evolution, leading to a general reduction in photosensory capability. It is thought that this event can be explained by the fact that this occurred during the time in which nocturnal mammals were evolving.[19]
Structure
[edit]The human melanopsin gene, opn4, is expressed in ipRGCs, which comprises only 1-2% of RGCs in the inner mammalian retina, as studied by Samer Hattar and colleagues.[9] The gene spans approximately 11.8 kb and is mapped to the long arm of chromosome 10. The gene includes nine introns and ten exons compared to the four to seven exons typically found in other human opsins.[16] In non-mammalian vertebrates, melanopsin is found in a wider subset of retinal cells, as well as in photosensitive structures outside the retina, such as the iris muscle of the eye, deep brain regions, the pineal gland, and the skin.[19] Paralogs of Opn4 include OPN1LW, OPN1MW, rhodopsin and encephalopsin.[21]
Melanopsin, like all other animal opsins (e.g. rhodopsin), is a G-protein-coupled receptor (GPCR). The melanopsin protein has an extarcellular N-terminal domain, an intracellular C-terminal domain, and seven alpha helices spanning through the plasma membrane.[14] The seventh helix has a lysine that corresponds to Lys2967.43 in cattle rhodopsin[14] and that is conserved in almost all opsins.[22] This lysine binds covalently retinal via a Schiff-base,[23][24] which makes melanopsin light sensitive. In fact this is abolished if the lysine is replaced by an alanine.[25]
Melanopsin is more closely related to invertebrate visual opsins, which are rhabdomeric opsin, than to vertebrate visual opsins, which are cliary opsins.[14][26][27] This is also reflected by the downstream signaling cascade, melanopsin couples in ipRGCs to the G-proteins G(q), G(11), and G(14), which are all of the G(q)-type.[28] In fact, they can functionally replace each other, as a knocking out only two of them has no phenotypical effect.[29] The G-proteins activate the phospholipase C PLCB4,[7] which causes the TRP-channels TRPC6 and TRPC7 mediate to open so that the cell depolarizes.[17][7] This is like in the photoreceptor cells of the Drosophila eye, and in contrast to the vertebrate rod and cone cells, where phototransduction eventually makes the cells hyperpolarize.[30] Like other rhabdomeric opsins, Melanopsin has intrinsic photoisomerase activity.[31]
Function
[edit]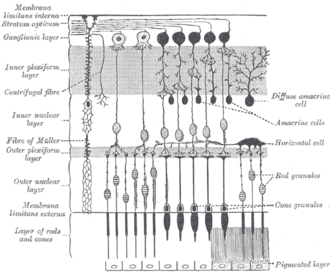
Melanopsin-containing ganglion cells,[32] like rods and cones, exhibit both light and dark adaptation; they adjust their sensitivity according to the recent history of light exposure.[33] However, while rods and cones are responsible for the reception of images, patterns, motion, and color, melanopsin-containing ipRGCs contribute to various reflexive responses of the brain and body to the presence of light.[17]
Evidence for melanopsin's physiological light detection has been tested in mice. A mouse cell line that is not normally photosensitive, Neuro-2a, is rendered light-sensitive by the addition of human melanopsin. The photoresponse is selectively sensitive to short-wavelength light (peak absorption ~479 nm),[34][35] and has an intrinsic photoisomerase regeneration function that is chromatically shifted to longer wavelengths.[36]
Melanopsin photoreceptors are sensitive to a range of wavelengths and reach peak light absorption at blue light wavelengths around 480 nanometers.[37] Other wavelengths of light activate the melanopsin signaling system with decreasing efficiency as they move away from the optimum 480 nm. For example, shorter wavelengths around 445 nm (closer to violet in the visible spectrum) are half as effective for melanopsin photoreceptor stimulation as light at 480 nm.[37]
Melanopsin in the iris of some, primarily nocturnal, mammals closes the iris when it is exposed to light. This local pupil light reflex (PLR) is absent from primates, even though their irises express melanopsin.[7]
Mechanism
[edit]When light with an appropriate frequency enters the eye, it activates the melanopsin contained in intrinsically photosensitive retinal ganglion cells (ipRGCs), triggering an action potential. These neuronal electrical signals travel through neuronal axons to specific brain targets, such as the center of pupillary control called the olivary pretectal nucleus (OPN) of the midbrain. Consequently, stimulation of melanopsin in ipRGCs mediates behavioral and physiological responses to light, such as pupil constriction and inhibition of melatonin release from the pineal gland.[38][39] The ipRGCs in the mammalian retina are one terminus of the retinohypothalamic tract that projects to the suprachiasmatic nucleus (SCN) of the hypothalamus. The suprachiasmatic nucleus is sometimes described as the brain's "master clock",[40] as it maintains the circadian rhythm, and nerve signals from ipRGCs to the SCN entrain the internal circadian rhythm to the rising and setting of the sun.[9] The SCN also receives input from rods and cones through the retinohypothalamic tract, so information from all three photosensitive cell types (rods, cones, and ipRGCs) in the mammalian retina are transmitted to the (SCN) SCN.[41]
Melanopsin-containing ganglion cells are thought to influence these targets by releasing the neurotransmitters glutamate and pituitary adenylate cyclase activating polypeptide (PACAP) from their axon terminals.[42] Melanopsin-containing ganglion cells also receive input from rods and cones that can add to the input to these pathways.
Effects on circadian rhythm
[edit]Melanopsin serves an important role in the photoentrainment of circadian rhythms in mammals. An organism that is photoentrained has aligned its activity to an approximately 24-hour cycle, the solar cycle on Earth.[43] In mammals, melanopsin expressing axons target the suprachiasmatic nucleus (SCN) through the retinohypothalamic tract (RHT).[9]
In mammals, the eye is the main photosensitive organ for the transmission of light signals to the brain. However, blind humans are still able to entrain to the environmental light-dark cycle, despite having no conscious perception of the light. One study exposed subjects to bright light for a prolonged duration of time and measured their melatonin concentrations. Melatonin was not only suppressed in visually unimpaired humans, but also in blind participants, suggesting that the photic pathway used by the circadian system is functionally intact despite blindness.[44] Therefore, physicians no longer practice enucleation of blind patients, or removal of the eyes at birth, since the eyes play a critical role in the photoentrainment of the circadian pacemaker.
In mutant breeds of mice that lacked only rods, only cones, or both rods and cones, all breeds of mice still entrained to changing light stimuli in the environment, but with a limited response, suggesting that rods and cones are not necessary for circadian photoentrainment and that the mammalian eye must have another photopigment required for the regulation of the circadian clock.[15]
Melanopsin-knockout mice display reduced photoentrainment. In comparison to wild-type mice that expressed melanopsin normally, deficits in light-induced phase shifts in locomotion activity were noted in melanopsin-null mice (Opn4 -/-).[18] These melanopsin-deficient mice did not completely lose their circadian rhythms, as they were still able to entrain to changing environmental stimuli, albeit more slowly than normal.[45] This indicated that, although melanopsin is sufficient for entrainment, it must work in conjunction with other photopigments for normal photoentrainment activity. Triple-mutant mice that were rod-less, cone-less, and melanopsin-less display a complete loss in the circadian rhythms, so all three photopigments in these photoreceptors, rhodopsin, photopsin and melanopsin, are necessary for photoentrainment.[46] Therefore, there is a functional redundancy between the three photopigments in the photoentrainment pathway of mammals. Deletion of only one photopigment does not eliminate the organism's ability to entrain to environmental light-dark cycles, but it does reduce the intensity of the response.
Regulation
[edit]Melanopsin undergoes phosphorylation on its intracellular carboxy tail as a way to deactivate its function. Compared to other opsins, melanopsin has an unusually long carboxy tail that contains 37 serine and threonine amino acid sites that could undergo phosphorylation.[47] However, a cluster of seven amino acids are sufficient to deactivate zebrafish melanopsin. These sites are dephosphorylated when melanopsin is exposed to light and are unique from those that regulate rhodopsin.[48] They are important for proper response to calcium ions in ipRGCs; lack of functional phosphorylation sites, particularly at serine-381 and serine-398, reduce the cell's response to light-induced calcium ion influx when voltage-gated calcium ion channels open.[49]
In terms of the gene Opn4, Dopamine (DA) is a factor in the regulation of melanopsin mRNA in ipRGCs.[50]
Clinical significance
[edit]The discovery of the role of melanopsin in non-image forming vision has led to a growth in optogenetics. This field has shown promise in clinical applications, including the treatment of human eye diseases such as retinitis pigmentosa and diabetes.[51] A missense mutation in Opn4, P10L, has been implicated in 5% of patients with Seasonal Affective Disorder (SAD).[10] This is a condition in which people experience depressive thoughts in the winter due to decreased available light. Additionally, a melanopsin based receptor has been linked to migraine pain.[52]
Restoration of vision
[edit]There has been recent research on the role of melanopsin in optogenetic therapy for patients with the degenerative eye disease retinitis pigmentosa (RP).[53] Reintroducing functional melanopsin into the eyes of mice with retinal degeneration restores the pupillary light reflex (PLR). These same mice could also distinguish light stimuli from dark stimuli and showed increased sensitivity to room light. The higher sensitivity demonstrated by these mice shows promise for vision restoration that may be applicable to humans and human eye diseases.[51][54]
Control of sleep/wake patterns
[edit]Melanopsin may aid in controlling sleep cycles and wakefulness. Tsunematsu and colleagues created transgenic mice that expressed melanopsin in hypothalamic orexin neurons. With a short 4-second pulse of blue light (guided by optical fibers), the transgenic mice could successfully transition from slow-wave sleep (SWS), which is commonly known as "deep sleep," to long-lasting wakefulness. After switching off the blue light, the hypothalamic orexin neurons showed activity for several tens of seconds.[51][55] It has been shown that rods and cones play no role in the onset of sleep by light, distinguishing them from ipRGCs and melanopsin. This provides strong evidence that there is a link between ipRGCs in humans and alertness, particularly with high frequency light (e.g. blue light). Therefore, melanopsin can be used as a therapeutic target for controlling the sleep-wake cycle.[56]
Regulation of blood glucose levels
[edit]In a paper published by Ye and colleagues in 2011, melanopsin was utilized to create an optogenetic synthetic transcription device that was tested in a therapeutic setting to produce Fc-glucagon-like peptide 1 (Fc-GLP-1), a fusion protein that helps control blood glucose levels in mammals with Type II Diabetes. The researchers subcutaneously implanted mice with microencapsulated transgenic HEK 293 cells that were cotransfected with two vectors including the melanopsin gene and the gene of interest under an NFAT (nuclear factor of activated T cells) promoter, respectively. It is through this engineered pathway that they successfully controlled the expression of Fc-GLP-1 in doubly recessive diabetic mice and reduced hyperglycemia, or high blood glucose levels, in these mice. This shows promise for the use of melanopsin as an optogenetic tool for the treatment of Type II diabetes.[51][57]
See also
[edit]- Light effects on circadian rhythm
- Opsins
- Intrinsically Photosensitive Retinal Ganglion Cells (ipRGCs)
- Suprachiasmatic nucleus (SCN)
- Retinohypothalamic tract
References
[edit]- ^ a b c GRCh38: Ensembl release 89: ENSG00000122375 – Ensembl, May 2017
- ^ a b c GRCm38: Ensembl release 89: ENSMUSG00000021799 – Ensembl, May 2017
- ^ "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ a b c Hankins MW, Peirson SN, Foster RG (January 2008). "Melanopsin: an exciting photopigment". Trends in Neurosciences. 31 (1): 27–36. doi:10.1016/j.tins.2007.11.002. PMID 18054803. S2CID 1645433.
- ^ Provencio I, Warthen DM (2012). "Melanopsin, the photopigment of intrinsically photosensitive retinal ganglion cells". Wiley Interdisciplinary Reviews: Membrane Transport and Signaling. 1 (2): 228–237. doi:10.1002/wmts.29.
- ^ a b c d Xue T, Do MT, Riccio A, Jiang Z, Hsieh J, Wang HC, et al. (November 2011). "Melanopsin signalling in mammalian iris and retina". Nature. 479 (7371): 67–73. Bibcode:2011Natur.479...67X. doi:10.1038/nature10567. PMC 3270891. PMID 22051675.
- ^ Angueyra JM, Pulido C, Malagón G, Nasi E, Gomez M (2012). "Melanopsin-expressing amphioxus photoreceptors transduce light via a phospholipase C signaling cascade". PLOS ONE. 7 (1): e29813. Bibcode:2012PLoSO...729813A. doi:10.1371/journal.pone.0029813. PMC 3250494. PMID 22235344.
- ^ a b c d Hattar S, Liao HW, Takao M, Berson DM, Yau KW (February 2002). "Melanopsin-containing retinal ganglion cells: architecture, projections, and intrinsic photosensitivity". Science. 295 (5557): 1065–1070. Bibcode:2002Sci...295.1065H. doi:10.1126/science.1069609. PMC 2885915. PMID 11834834.
- ^ a b Roecklein KA, Rohan KJ, Duncan WC, Rollag MD, Rosenthal NE, Lipsky RH, et al. (April 2009). "A missense variant (P10L) of the melanopsin (OPN4) gene in seasonal affective disorder". Journal of Affective Disorders. 114 (1–3): 279–285. doi:10.1016/j.jad.2008.08.005. PMC 2647333. PMID 18804284.
- ^ Nissilä J, Mänttäri S, Tuominen H, Särkioja T, Takala T, Saarela S, et al. (2012). "P-780 – The abundance and distribution of melanopsin (OPN4) protein in human brain". European Psychiatry. 27: 1–8. doi:10.1016/S0924-9338(12)74947-7. S2CID 82045589.
- ^ Dkhissi-Benyahya O, Rieux C, Hut RA, Cooper HM (April 2006). "Immunohistochemical evidence of a melanopsin cone in human retina". Investigative Ophthalmology & Visual Science. 47 (4): 1636–1641. doi:10.1167/iovs.05-1459. PMID 16565403.
- ^ Horiguchi H, Winawer J, Dougherty RF, Wandell BA (January 2013). "Human trichromacy revisited". Proceedings of the National Academy of Sciences of the United States of America. 110 (3): E260–E269. Bibcode:2013PNAS..110E.260H. doi:10.1073/pnas.1214240110. PMC 3549098. PMID 23256158.
- ^ a b c d Provencio I, Jiang G, De Grip WJ, Hayes WP, Rollag MD (January 1998). "Melanopsin: An opsin in melanophores, brain, and eye". Proceedings of the National Academy of Sciences of the United States of America. 95 (1): 340–345. Bibcode:1998PNAS...95..340P. doi:10.1073/pnas.95.1.340. PMC 18217. PMID 9419377.
- ^ a b Freedman MS, Lucas RJ, Soni B, von Schantz M, Muñoz M, David-Gray Z, et al. (April 1999). "Regulation of mammalian circadian behavior by non-rod, non-cone, ocular photoreceptors". Science. 284 (5413): 502–504. Bibcode:1999Sci...284..502F. doi:10.1126/science.284.5413.502. PMID 10205061.
- ^ a b c Provencio I, Rodriguez IR, Jiang G, Hayes WP, Moreira EF, Rollag MD (January 2000). "A novel human opsin in the inner retina". The Journal of Neuroscience. 20 (2): 600–605. doi:10.1523/JNEUROSCI.20-02-00600.2000. PMC 6772411. PMID 10632589.
- ^ a b c Berson DM, Dunn FA, Takao M (February 2002). "Phototransduction by retinal ganglion cells that set the circadian clock". Science. 295 (5557): 1070–1073. Bibcode:2002Sci...295.1070B. doi:10.1126/science.1067262. PMID 11834835. S2CID 30745140.
- ^ a b Panda S, Sato TK, Castrucci AM, Rollag MD, DeGrip WJ, Hogenesch JB, et al. (December 2002). "Melanopsin (Opn4) requirement for normal light-induced circadian phase shifting". Science. 298 (5601): 2213–2216. Bibcode:2002Sci...298.2213P. doi:10.1126/science.1076848. PMID 12481141. S2CID 20602808.
- ^ a b c Bellingham J, Chaurasia SS, Melyan Z, Liu C, Cameron MA, Tarttelin EE, et al. (July 2006). "Evolution of melanopsin photoreceptors: discovery and characterization of a new melanopsin in nonmammalian vertebrates". PLOS Biology. 4 (8): e254. doi:10.1371/journal.pbio.0040254. PMC 1514791. PMID 16856781.

- ^ Benton MJ (May 1990). "Phylogeny of the major tetrapod groups: morphological data and divergence dates". Journal of Molecular Evolution. 30 (5): 409–424. Bibcode:1990JMolE..30..409B. doi:10.1007/BF02101113. PMID 2111854. S2CID 35082873.
- ^ Terakita A (1 March 2005). "The opsins". Genome Biology. 6 (3): 213. doi:10.1186/gb-2005-6-3-213. PMC 1088937. PMID 15774036.
- ^ Gühmann M, Porter ML, Bok MJ (August 2022). "The Gluopsins: Opsins without the Retinal Binding Lysine". Cells. 11 (15): 2441. doi:10.3390/cells11152441. PMC 9368030. PMID 35954284.
- ^ Collins FD (March 1953). "Rhodopsin and indicator yellow". Nature. 171 (4350): 469–471. Bibcode:1953Natur.171..469C. doi:10.1038/171469a0. PMID 13046517. S2CID 4152360.
- ^ Pitt GA, Collins FD, Morton RA, Stok P (January 1955). "Studies on rhodopsin. VIII. Retinylidenemethylamine, an indicator yellow analogue". The Biochemical Journal. 59 (1): 122–128. doi:10.1042/bj0590122. PMC 1216098. PMID 14351151.
- ^ Kumbalasiri T, Rollag MD, Isoldi MC, Castrucci AM, Provencio I (March 2007). "Melanopsin triggers the release of internal calcium stores in response to light". Photochemistry and Photobiology. 83 (2): 273–279. doi:10.1562/2006-07-11-RA-964. PMID 16961436. S2CID 23060331.
- ^ Porter ML, Blasic JR, Bok MJ, Cameron EG, Pringle T, Cronin TW, et al. (January 2012). "Shedding new light on opsin evolution". Proceedings. Biological Sciences. 279 (1726): 3–14. doi:10.1098/rspb.2011.1819. PMC 3223661. PMID 22012981.
- ^ Ramirez MD, Pairett AN, Pankey MS, Serb JM, Speiser DI, Swafford AJ, et al. (26 October 2016). "The last common ancestor of most bilaterian animals possessed at least 9 opsins". Genome Biology and Evolution: evw248. doi:10.1093/gbe/evw248. PMC 5521729. PMID 27797948.
- ^ Hughes S, Jagannath A, Hickey D, Gatti S, Wood M, Peirson SN, et al. (January 2015). "Using siRNA to define functional interactions between melanopsin and multiple G Protein partners". Cellular and Molecular Life Sciences. 72 (1): 165–179. doi:10.1007/s00018-014-1664-6. PMC 4282707. PMID 24958088.
- ^ Chew KS, Schmidt TM, Rupp AC, Kofuji P, Trimarchi JM (28 May 2014). "Loss of gq/11 genes does not abolish melanopsin phototransduction". PLOS ONE. 9 (5): e98356. Bibcode:2014PLoSO...998356C. doi:10.1371/journal.pone.0098356. PMC 4037210. PMID 24870805.
- ^ Sexton T, Buhr E, Van Gelder RN (January 2012). "Melanopsin and mechanisms of non-visual ocular photoreception". The Journal of Biological Chemistry. 287 (3): 1649–1656. doi:10.1074/jbc.r111.301226. PMC 3265846. PMID 22074930.
- ^ Panda S, Nayak SK, Campo B, Walker JR, Hogenesch JB, Jegla T (January 2005). "Illumination of the melanopsin signaling pathway". Science. 307 (5709): 600–604. Bibcode:2005Sci...307..600P. doi:10.1126/science.1105121. PMID 15681390. S2CID 22713904.
- ^ Feigl B, Zele AJ (August 2014). "Melanopsin-expressing intrinsically photosensitive retinal ganglion cells in retinal disease" (PDF). Optometry and Vision Science. 91 (8): 894–903. doi:10.1097/OPX.0000000000000284. PMID 24879087. S2CID 34057255.
- ^ Wong KY, Dunn FA, Berson DM (December 2005). "Photoreceptor adaptation in intrinsically photosensitive retinal ganglion cells". Neuron. 48 (6): 1001–1010. doi:10.1016/j.neuron.2005.11.016. PMID 16364903.
- ^ Bailes HJ, Lucas RJ (May 2013). "Human melanopsin forms a pigment maximally sensitive to blue light (λmax ≈ 479 nm) supporting activation of G(q/11) and G(i/o) signalling cascades". Proceedings. Biological Sciences. 280 (1759): 20122987. doi:10.1098/rspb.2012.2987. PMC 3619500. PMID 23554393.
- ^ Berson DM (August 2007). "Phototransduction in ganglion-cell photoreceptors". Pflügers Archiv. 454 (5): 849–855. doi:10.1007/s00424-007-0242-2. PMID 17351786.
- ^ Melyan Z, Tarttelin EE, Bellingham J, Lucas RJ, Hankins MW (February 2005). "Addition of human melanopsin renders mammalian cells photoresponsive". Nature. 433 (7027): 741–745. Bibcode:2005Natur.433..741M. doi:10.1038/nature03344. PMID 15674244. S2CID 4426682.
- ^ a b Enezi J, Revell V, Brown T, Wynne J, Schlangen L, Lucas R (August 2011). "A "melanopic" spectral efficiency function predicts the sensitivity of melanopsin photoreceptors to polychromatic lights". Journal of Biological Rhythms. 26 (4): 314–323. doi:10.1177/0748730411409719. PMID 21775290. S2CID 22369861.
- ^ Markwell EL, Feigl B, Zele AJ (May 2010). "Intrinsically photosensitive melanopsin retinal ganglion cell contributions to the pupillary light reflex and circadian rhythm". Clinical & Experimental Optometry. 93 (3): 137–149. doi:10.1111/j.1444-0938.2010.00479.x. PMID 20557555. S2CID 21778407.
- ^ Zaidi FH, Hull JT, Peirson SN, Wulff K, Aeschbach D, Gooley JJ, et al. (December 2007). "Short-wavelength light sensitivity of circadian, pupillary, and visual awareness in humans lacking an outer retina". Current Biology. 17 (24): 2122–2128. Bibcode:2007CBio...17.2122Z. doi:10.1016/j.cub.2007.11.034. PMC 2151130. PMID 18082405.
- ^ Evans JA (July 2016). "Collective timekeeping among cells of the master circadian clock". The Journal of Endocrinology. 230 (1): R27–R49. doi:10.1530/JOE-16-0054. PMC 4938744. PMID 27154335.
- ^ Reppert SM, Weaver DR (August 2002). "Coordination of circadian timing in mammals". Nature. 418 (6901): 935–941. Bibcode:2002Natur.418..935R. doi:10.1038/nature00965. PMID 12198538. S2CID 4430366.
- ^ Hannibal J, Fahrenkrug J (April 2004). "Target areas innervated by PACAP-immunoreactive retinal ganglion cells". Cell and Tissue Research. 316 (1): 99–113. doi:10.1007/s00441-004-0858-x. PMID 14991397. S2CID 24148323.
- ^ Allada R, Emery P, Takahashi JS, Rosbash M (2001). "Stopping time: the genetics of fly and mouse circadian clocks". Annual Review of Neuroscience. 24 (1): 1091–1119. doi:10.1146/annurev.neuro.24.1.1091. PMID 11520929.
- ^ Czeisler CA, Shanahan TL, Klerman EB, Martens H, Brotman DJ, Emens JS, et al. (January 1995). "Suppression of melatonin secretion in some blind patients by exposure to bright light". The New England Journal of Medicine. 332 (1): 6–11. doi:10.1056/NEJM199501053320102. PMID 7990870.
- ^ Rollag MD, Berson DM, Provencio I (June 2003). "Melanopsin, ganglion-cell photoreceptors, and mammalian photoentrainment". Journal of Biological Rhythms. 18 (3): 227–234. doi:10.1177/0748730403018003005. PMID 12828280. S2CID 9034442.
- ^ Panda S, Provencio I, Tu DC, Pires SS, Rollag MD, Castrucci AM, et al. (July 2003). "Melanopsin is required for non-image-forming photic responses in blind mice". Science. 301 (5632): 525–527. Bibcode:2003Sci...301..525P. doi:10.1126/science.1086179. PMID 12829787. S2CID 37600812.
- ^ Blasic JR, Lane Brown R, Robinson PR (May 2012). "Light-dependent phosphorylation of the carboxy tail of mouse melanopsin". Cellular and Molecular Life Sciences. 69 (9): 1551–1562. doi:10.1007/s00018-011-0891-3. PMC 4045631. PMID 22159583.
- ^ Blasic JR, Matos-Cruz V, Ujla D, Cameron EG, Hattar S, Halpern ME, et al. (April 2014). "Identification of critical phosphorylation sites on the carboxy tail of melanopsin". Biochemistry. 53 (16): 2644–2649. doi:10.1021/bi401724r. PMC 4010260. PMID 24678795.
- ^ Fahrenkrug J, Falktoft B, Georg B, Hannibal J, Kristiansen SB, Klausen TK (December 2014). "Phosphorylation of rat melanopsin at Ser-381 and Ser-398 by light/dark and its importance for intrinsically photosensitive ganglion cells (ipRGCs) cellular Ca2+ signaling". The Journal of Biological Chemistry. 289 (51): 35482–35493. doi:10.1074/jbc.M114.586529. PMC 4271233. PMID 25378407.
- ^ Sakamoto K, Liu C, Kasamatsu M, Pozdeyev NV, Iuvone PM, Tosini G (December 2005). "Dopamine regulates melanopsin mRNA expression in intrinsically photosensitive retinal ganglion cells". The European Journal of Neuroscience. 22 (12): 3129–3136. doi:10.1111/j.1460-9568.2005.04512.x. PMID 16367779. S2CID 21517576.
- ^ a b c d Koizumi A, Tanaka KF, Yamanaka A (January 2013). "The manipulation of neural and cellular activities by ectopic expression of melanopsin". Neuroscience Research. 75 (1): 3–5. doi:10.1016/j.neures.2012.07.010. PMID 22982474. S2CID 21771987.
- ^ Jennifer Couzin-Frankel (2010). "Why Light Makes Migraines Worse – ScienceNOW". Archived from the original on 31 July 2016. Retrieved 3 April 2011.
- ^ Busskamp V, Picaud S, Sahel JA, Roska B (February 2012). "Optogenetic therapy for retinitis pigmentosa". Gene Therapy. 19 (2): 169–175. doi:10.1038/gt.2011.155. PMID 21993174.
- ^ Lin B, Koizumi A, Tanaka N, Panda S, Masland RH (October 2008). "Restoration of visual function in retinal degeneration mice by ectopic expression of melanopsin". Proceedings of the National Academy of Sciences of the United States of America. 105 (41): 16009–16014. Bibcode:2008PNAS..10516009L. doi:10.1073/pnas.0806114105. PMC 2572922. PMID 18836071.
- ^ Tsunematsu T, Tanaka KF, Yamanaka A, Koizumi A (January 2013). "Ectopic expression of melanopsin in orexin/hypocretin neurons enables control of wakefulness of mice in vivo by blue light". Neuroscience Research. 75 (1): 23–28. doi:10.1016/j.neures.2012.07.005. PMID 22868039. S2CID 207152803.
- ^ Lupi D, Oster H, Thompson S, Foster RG (September 2008). "The acute light-induction of sleep is mediated by OPN4-based photoreception". Nature Neuroscience. 11 (9): 1068–1073. doi:10.1038/nn.2179. hdl:11858/00-001M-0000-0012-DD96-A. PMID 19160505. S2CID 15941500.
- ^ Ye H, Daoud-El Baba M, Peng RW, Fussenegger M (June 2011). "A synthetic optogenetic transcription device enhances blood-glucose homeostasis in mice". Science. 332 (6037): 1565–1568. Bibcode:2011Sci...332.1565Y. doi:10.1126/science.1203535. PMID 21700876. S2CID 6166189.
Further reading
[edit]- Rovere G, Nadal-Nicolás FM, Wang J, Bernal-Garro JM, García-Carrillo N, Villegas-Pérez MP, et al. (December 2016). "Melanopsin-Containing or Non-Melanopsin-Containing Retinal Ganglion Cells Response to Acute Ocular Hypertension With or Without Brain-Derived Neurotrophic Factor Neuroprotection". Investigative Ophthalmology & Visual Science. 57 (15): 6652–6661. doi:10.1167/iovs.16-20146. PMID 27930778.




